Abstract
Purpose
Sepsis in critically ill patients with injury bears a high morbidity and mortality. Extensive phenotypic monitoring of leucocyte subsets in critically ill patients at ICU admission and during sepsis development is still scarce. The main objective of this study was to identify early changes in leukocyte phenotype which would correlate with later development of sepsis.
Methods
Patients who were admitted in a tertiary ICU for organ support after severe injury (elective cardiac surgery, trauma, necessity of prolonged ventilation or stroke) were sampled on admission (T1) and 48–72 h later (T2) for phenotyping of leukocyte subsets by flow cytometry and cytokines measurements. Those who developed secondary sepsis or septic shock were sampled again on the day of sepsis diagnosis (Tx).
Results
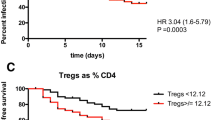
Ninety-nine patients were included in the final analysis. Nineteen (19.2%) patients developed secondary sepsis or septic shock. They presented significantly higher absolute monocyte counts and CRP at T1 compared to non-septic patients (1030/µl versus 550/µl, p = 0.013 and 5.1 mg/ml versus 2.5 mg/ml, p = 0.046, respectively). They also presented elevated levels of monocytes with low expression of L-selectin (CD62Lneg monocytes) (OR[95%CI] 4.5 (1.4–14.5), p = 0.01) and higher SOFA score (p < 0.0001) at T1 and low mHLA-DR at T2 (OR[95%CI] 0.003 (0.00–0.17), p = 0.049). Stepwise logistic regression analysis showed that both monocyte markers and high SOFA score (> 8) were independently associated with nosocomial sepsis occurrence. No other leucocyte count or surface marker nor any cytokine measurement correlated with sepsis occurrence.
Conclusion
Monocyte counts and change of phenotype are associated with secondary sepsis occurrence in critically ill patients with injury.


Similar content being viewed by others
Availability of data and materials
The datasets used and/or analysed (beyond those included in the supplementary files) during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- BSI:
-
Blood stream infection
- CI:
-
Confidence interval
- CLABSI:
-
Central line associated blood stream infection
- CRP:
-
C-reactive protein
- HAP:
-
Hospital-acquired pneumonia
- HLA-DR:
-
Human leucocyte antigen
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- LOS:
-
Length of stay
- MFI:
-
Median fluorescence index or median of fluorescence intensity
- OR:
-
Odds ratio
- PE:
-
Phycoerythrin-linked
- PerCP:
-
Perinidin-chlorophyll protein-linked
- ROC:
-
Receiver operating characteristic
- SOFA:
-
Sequential organ failure assessment
- SSTI:
-
Surgical site and soft tissue infection
- TNF-α:
-
Tumor necrosis factor α
- VAP:
-
Ventilator-associated pneumonia
References
Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, Finfer S, Pelosi P, Brazzi L, Aditianingsih D, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323:1478–87.
Markwart R, Saito H, Harder T, Tomczyk S, Cassini A, Fleischmann-Struzek C, Reichert F, Eckmanns T, Allegranzi B. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46:1536–51.
Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–6.
Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Namendys-Silva SA, Martin-Loeches I, Leone M, Lupu MN, Vincent JL, et al. Sepsis in intensive care unit patients: worldwide data from the intensive care over nations audit. Open Forum Infect Dis. 2018;5:ofy313.
Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–501.
Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. Immunosuppression following surgical and traumatic injury. Surg Today. 2010;40:793–808.
Angele MK, Chaudry IH. Surgical trauma and immunosuppression: pathophysiology and potential immunomodulatory approaches. Langenbecks Arch Surg. 2005;390:333–41.
Timmermans K, Kox M, Vaneker M, van den Berg M, John A, van Laarhoven A, van der Hoeven H, Scheffer GJ, Pickkers P. Plasma levels of danger-associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med. 2016;42:551–61.
Slade MS, Simmons RL, Yunis E, Greenberg LJ. Immunodepression after major surgery in normal patients. Surgery. 1975;78:363–72.
Munster AM, Eurenius K, Katz RM, Canales L, Foley FD, Mortensen RF. Cell-mediated immunity after thermal injury. Ann Surg. 1973;177:139–43.
Conway Morris A, Kefala K, Wilkinson TS, Dhaliwal K, Farrell L, Walsh T, Mackenzie SJ, Reid H, Davidson DJ, Haslett C, et al. C5a mediates peripheral blood neutrophil dysfunction in critically ill patients. Am J Respir Crit Care Med. 2009;180:19–28.
Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–8.
Venet F, Tissot S, Debard AL, Faudot C, Crampe C, Pachot A, Ayala A, Monneret G. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: correlation with severity and secondary septic shock. Crit Care Med. 2007;35:1910–7.
Venet F, Chung CS, Monneret G, Huang X, Horner B, Garber M, Ayala A. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2008;83:523–35.
Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–44.
Ditschkowski M, Kreuzfelder E, Majetschak M, Obertacke U, Schade UF, Grosse-Wilde H. Reduced B cell HLA-DR expression and natural killer cell counts in patients prone to sepsis after injury. Eur J Surg. 1999;165:1129–33.
Livingston DH, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC Jr. Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 1988;123:1309–12.
Asadullah K, Woiciechowsky C, Docke WD, Liebenthal C, Wauer H, Kox W, Volk HD, Vogel S, Von Baehr R. Immunodepression following neurosurgical procedures. Crit Care Med. 1995;23:1976–83.
Bidar F, Bodinier M, Venet F, Lukaszewicz AC, Brengel-Pesce K, Conti F, Quemeneur L, Leissner P, Tan LK, Textoris J, et al. Concomitant assessment of monocyte HLA-DR expression and ex vivo TNF-alpha release as markers of adverse outcome after various injuries-insights from the REALISM study. J Clin Med. 2021;11:96.
Gouel-Cheron A, Allaouchiche B, Guignant C, Davin F, Floccard B, Monneret G. Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS ONE. 2012;7: e33095.
Lukaszewicz AC, Grienay M, Resche-Rigon M, Pirracchio R, Faivre V, Boval B, Payen D. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37:2746–52.
Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–81.
Polk HC Jr, Cheadle WG, Livingston DH, Rodriguez JL, Starko KM, Izu AE, Jaffe HS, Sonnenfeld G. A randomized prospective clinical trial to determine the efficacy of interferon-gamma in severely injured patients. Am J Surg. 1992;163:191–6.
Dries DJ, Jurkovich GJ, Maier RV, Clemmer TP, Struve SN, Weigelt JA, Stanford GG, Herr DL, Champion HR, Lewis FR, et al. Effect of interferon gamma on infection-related death in patients with severe injuries. A randomized, double-blind, placebo-controlled trial. Arch Surg. 1994;129:1031–41 (discussion 1042).
Conway Morris A, Datta D, Shankar-Hari M, Stephen J, Weir CJ, Rennie J, Antonelli J, Bateman A, Warner N, Judge K, et al. Cell-surface signatures of immune dysfunction risk-stratify critically ill patients: INFECT study. Intensive Care Med. 2018;44:627–35.
Guerin E, Orabona M, Raquil MA, Giraudeau B, Bellier R, Gibot S, Bene MC, Lacombe F, Droin N, Solary E, et al. Circulating immature granulocytes with T-cell killing functions predict sepsis deterioration*. Crit Care Med. 2014;42:2007–18.
Daix T, Guerin E, Tavernier E, Mercier E, Gissot V, Herault O, Mira JP, Dumas F, Chapuis N, Guitton C, et al. Multicentric standardized flow cytometry routine assessment of patients with sepsis to predict clinical worsening. Chest. 2018;154:617–27.
Venet F, Textoris J, Blein S, Rol ML, Bodinier M, Canard B, Cortez P, Meunier B, Tan LK, Tipple C, et al. Immune profiling demonstrates a common immune signature of delayed acquired immunodeficiency in patients with various etiologies of severe injury. Crit Care Med. 2022;50:565–75.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
Boev C, Kiss E. Hospital-acquired infections: current trends and prevention. Crit Care Nurs Clin North Am. 2017;29:51–65.
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratalà J, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases Society of America and the American thoracic society. Clin Infect Dis. 2016;63:e61–111.
Novosad SA, Fike L, Dudeck MA, Allen-Bridson K, Edwards JR, Edens C, Sinkowitz-Cochran R, Powell K, Kuhar D. Pathogens causing central-line-associated bloodstream infections in acute-care hospitals-United States, 2011–2017. Infect Control Hosp Epidemiol. 2020;41:313–9.
Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep. 2015;5:13886.
Venet F, Guignant C, Monneret G. Flow cytometry developments and perspectives in clinical studies: examples in ICU patients. Methods Mol Biol. 2011;761:261–75.
Agnello L, Giglio RV, Bivona G, Scazzone C, Gambino CM, Iacona A, Ciaccio AM, Lo Sasso B, Ciaccio M. The value of a complete blood count (CBC) for sepsis diagnosis and prognosis. Diagnostics (Basel). 2021;11:1881.
Radzyukevich YV, Kosyakova NI, Prokhorenko IR. Participation of monocyte subpopulations in progression of experimental endotoxemia (EE) and systemic inflammation. J Immunol Res. 2021;2021:1762584.
Coiffard B, Diallo AB, Culver A, Mezouar S, Hammad E, Vigne C, Nicolino-Brunet C, Dignat-George F, Baumstarck K, Boucekine M, et al. Circadian rhythm disruption and sepsis in severe trauma patients. Shock. 2019;52:29–36.
Dong X, Wang C, Liu X, Bai X, Li Z. The trajectory of alterations in immune-cell counts in severe-trauma patients is related to the later occurrence of sepsis and mortality: retrospective study of 917 cases. Front Immunol. 2020;11: 603353.
Chung H, Lee JH, Jo YH, Hwang JE, Kim J. Circulating monocyte counts and its impact on outcomes in patients with severe sepsis including septic shock. Shock. 2019;51:423–9.
Aydin M, Barut S, Akbulut HH, Ucar S, Orman A. Application of flow cytometry in the early diagnosis of neonatal sepsis. Ann Clin Lab Sci. 2017;47:184–90.
Buhrer C, Graulich J, Stibenz D, Dudenhausen JW, Obladen M. L-selectin is down-regulated in umbilical cord blood granulocytes and monocytes of newborn infants with acute bacterial infection. Pediatr Res. 1994;36:799–804.
Genel F, Atlihan F, Gulez N, Kazanci E, Vergin C, Terek DT, Yurdun OC. Evaluation of adhesion molecules CD64, CD11b and CD62L in neutrophils and monocytes of peripheral blood for early diagnosis of neonatal infection. World J Pediatr. 2012;8:72–5.
Griffin JD, Spertini O, Ernst TJ, Belvin MP, Levine HB, Kanakura Y, Tedder TF. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. J Immunol. 1990;145:576–84.
Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–41.
Spertini O, Luscinskas FW, Gimbrone MA Jr, Tedder TF. Monocyte attachment to activated human vascular endothelium in vitro is mediated by leukocyte adhesion molecule-1 (L-selectin) under nonstatic conditions. J Exp Med. 1992;175:1789–92.
Spertini O, Kansas GS, Munro JM, Griffin JD, Tedder TF. Regulation of leukocyte migration by activation of the leukocyte adhesion molecule-1 (LAM-1) selectin. Nature. 1991;349:691–4.
Holland J, Carey M, Hughes N, Sweeney K, Byrne PJ, Healy M, Ravi N, Reynolds JV. Intraoperative splanchnic hypoperfusion, increased intestinal permeability, down-regulation of monocyte class II major histocompatibility complex expression, exaggerated acute phase response, and sepsis. Am J Surg. 2005;190:393–400.
Cocks RA, Chan TY, Rainer TH. Leukocyte L-selectin is up-regulated after mechanical trauma in adults. J Trauma. 1998;45:1–6.
Briggs GD, Lemmert K, Lott NJ, de Malmanche T, Balogh ZJ. Biomarkers to guide the timing of surgery: neutrophil and monocyte L-selectin predict postoperative sepsis in orthopaedic trauma patients. J Clin Med. 2021;10:2207.
Rouget C, Girardot T, Textoris J, Monneret G, Rimmele T, Venet F. Biological markers of injury-induced immunosuppression. Minerva Anestesiol. 2017;83:302–14.
Huang LF, Yao YM, Dong N, Yu Y, He LX, Sheng ZY. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Crit Care. 2010;14:R3.
Venet F, Chung CS, Kherouf H, Geeraert A, Malcus C, Poitevin F, Bohe J, Lepape A, Ayala A, Monneret G. Increased circulating regulatory T cells (CD4(+)CD25 (+)CD127 (-)) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009;35:678–86.
Sebastian A, Sanju S, Jain P, Priya VV, Varma PK, Mony U. Non-classical monocytes and its potential in diagnosing sepsis post cardiac surgery. Int Immunopharmacol. 2021;99: 108037.
Matuschak GM. Circulating cytokine concentrations and outcome prediction in intensive care unit patients: still the tip of the iceberg? Crit Care Med. 1996;24:1769–71.
O’Dwyer MJ, Owen HC, Torrance HD. The perioperative immune response. Curr Opin Crit Care. 2015;21:336–42.
Cid J, Aguinaco R, Sanchez R, Garcia-Pardo G, Llorente A. Neutrophil CD64 expression as marker of bacterial infection: a systematic review and meta-analysis. J Infect. 2010;60:313–9.
Gamez-Diaz LY, Enriquez LE, Matute JD, Velasquez S, Gomez ID, Toro F, Ospina S, Bedoya V, Arango CM, Valencia ML, et al. Diagnostic accuracy of HMGB-1, sTREM-1, and CD64 as markers of sepsis in patients recently admitted to the emergency department. Acad Emerg Med. 2011;18:807–15.
Gros A, Roussel M, Sauvadet E, Gacouin A, Marque S, Chimot L, Lavoue S, Camus C, Fest T, Le Tulzo Y. The sensitivity of neutrophil CD64 expression as a biomarker of bacterial infection is low in critically ill patients. Intensive Care Med. 2012;38:445–52.
Icardi M, Erickson Y, Kilborn S, Stewart B, Grief B, Scharnweber G. CD64 index provides simple and predictive testing for detection and monitoring of sepsis and bacterial infection in hospital patients. J Clin Microbiol. 2009;47:3914–9.
Layios N, Lambermont B, Canivet JL, Morimont P, Preiser JC, Garweg C, Ledoux D, Frippiat F, Piret S, Giot JB, et al. Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Crit Care Med. 2012;40:2304–9.
Conway Morris A, Anderson N, Brittan M, Wilkinson TS, McAuley DF, Antonelli J, McCulloch C, Barr LC, Dhaliwal K, Jones RO, et al. Combined dysfunctions of immune cells predict nosocomial infection in critically ill patients. Br J Anaesth. 2013;111:778–87.
Bourgoin P, Taspinar R, Gossez M, Venet F, Delwarde B, Rimmele T, Morange PE, Malergue F, Monneret G. Toward monocyte HLA-DR bedside monitoring: a proof-of-concept study. Shock. 2021;55:782–9.
Acknowledgements
We are grateful for the insightful revision of Profs B. Misset and JM Cavaillon. We are thankful to BD Sciences for the generous gift of monoclonal antibodies.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NL, PD, CO and AG designed the study; NL, CG, CD, CL, AH and AG did the experiments; NL, CG, NM and AG analysed the data; NL wrote the manuscript, CG and AG revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
The study was appointed the Belgian number B707201111981 by the local ethics committee of University Hospital of Liège (number 707) and written informed consent was obtained from the patient or his/her legal representative.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.

15010_2023_1983_MOESM1_ESM.jpg
Supplementary file1 Figure S1 (A-J) Flow cytometry gating strategy used to define lymphocyte, monocyte and granulocyte subsets by flow cytometry (explanation in the text). (JPG 246 KB)

15010_2023_1983_MOESM2_ESM.jpg
Supplementary file2 Figure S2 Panel A: Measurements at ICU admission in nonseptic and septic patients and in healthy controls (> 50 years). (*: p<0.05) (JPG 90 KB)

15010_2023_1983_MOESM3_ESM.jpg
Supplementary file3 Figure S2 Panel B: Predictive value of monocyte absolute count (/µl) obtained at T1. ROC curve analysis of sepsis occurrence based on levels of monocytes and of SOFA is shown. (JPG 109 KB)

15010_2023_1983_MOESM4_ESM.jpg
Supplementary file4 Figure S3 Absolute count of CD62Lneg monocytes (/µl): evolution of septic patients (N=12 patients with measurement at ICU admission, 48 to 72h later and on the day of sepsis diagnosis). (ns: not statistically significant). (JPG 86 KB)

15010_2023_1983_MOESM5_ESM.jpg
Supplementary file5 Figure S4 Intermediate monocytes (CD14++CD16+) median HLA-DR (MFI): evolution of septic patients (N=7 with measurement at ICU admission, 48 to 72h later and on the day of sepsis diagnosis). (ns: not statistically significant). (JPG 87 KB)
15010_2023_1983_MOESM6_ESM.docx
Supplementary file6 Table S1 Sites of infection and microbiological documentation. HAP-VAP: hospital-acquired pneumonia. VAP: ventilator-associated pneumonia. SSTI: surgical site and soft tissue infection. CLABSI: central line associated blood stream infection. BSI: primary blood stream infection. Some patients developed more than one infection and some infections were polymicrobial. Two episodes of VAP were clinically diagnosed and empirically treated although no organism grew in culture. (DOCX 17 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Layios, N., Gosset, C., Maes, N. et al. Prospective flow cytometry analysis of leucocyte subsets in critically ill patients who develop sepsis: a pilot study. Infection 51, 1305–1317 (2023). https://doi.org/10.1007/s15010-023-01983-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-023-01983-3