Abstract
Introduction
There are rising evidences that subcortical structures, including the basal ganglia, are affected in patients with epilepsy. These structures are thought to influence the modulation and phenotypic expression of epileptic seizures. Our study aimed to evaluate the presence of structural abnormalities in subcortical structures in patients with juvenile myoclonic epilepsy (JME).
Methods
This cross-sectional study included 51 patients who were diagnosed with JME and who were monitored on an outpatient basis at the Clinic for Neurology and Psychiatry for Children and Youth in Belgrade from January 1985 to October 2017. All patients underwent transcranial parenchymal sonography (TCS) from October 2015 to October 2017. Relation of clinical parameters (seizure control andcognitive functioning,) with TCS results was assessed.
Results
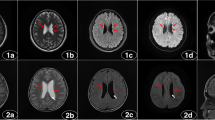
Hyperechogenicity of the substantia nigra (SN) was detected in 37.2% of JME subjects and it was significantly more common in patients with JME than in the control group. The marked echogenicity of the red nucleus (RN) was detected in 17.6% of cases, while 11.8% of subjects had hyperechogenic RN. The presence of hyperechogenic RN (both right and left) was significantly more frequent in the group of patients with JME compared to the control group. The third ventricle diameter was larger in patients with JME than in controls.
Conclusion
Structural changes of certain subcortical structures, primarily SN and RN, detected in JME patients indicate additional non-lesional abnormalities of the basal ganglia and midbrain structures in these patients.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to concerns regarding the privacy of study participants.
References
Commission on Classification and Terminology of the International League Against Epilepsy (1989) Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 30:389–399. https://doi.org/10.1111/j.1528-1157.1989.tb05316.x
Crespel A, Gelisse P, Reed RC, Ferlazzo E, Jerney J, Schmitz B, Genton P (2013) Management of juvenile myoclonic epilepsy. Epilepsy Behav 28(Suppl 1):S81-86. https://doi.org/10.1016/j.yebeh.2013.01.001
Genton P, Thomas P, Kasteleijn-Nolst Trenité DGA, Medina MT, Salas-Puig J (2013) Clinical aspects of juvenile myoclonic epilepsy. Epilepsy Behav 28(Suppl 1):S8-14. https://doi.org/10.1016/j.yebeh.2012.10.034
Baykan B, Peter Wolf P (2017) Juvenile myoclonic epilepsy as a spectrum disorder: a focused review. Seizure 49:36–41. https://doi.org/10.1016/j.seizure.2017.05.011
Saini J, Sinha S, Bagepally BS, Ramchandraiah CT, Thennarasu K, Prasad C, Taly AB, Satishchandra P (2013) Subcortical structural abnormalities in juvenile myoclonic epilepsy (JME): MR volumetry and vertex based analysis. Seizure 22(3):230–235. https://doi.org/10.1016/j.seizure.2013.01.001
Hattingen E, Lückerath C, Pellikan S, Vronski D, Roth C, Knake S, Kieslich M, Pilatus U (2014) Frontal and thalamic changes of GABA concentrationindicate dysfunction of thalamofrontal networks in juvenilemyoclonic epilepsy. Epilepsia 55(7):1030–1037. https://doi.org/10.1111/epi.12656
Landvogt C, Buchholz HG, Bernedo V, Schreckenberger M, Werhahn KJ (2010) Alteration of dopamine D2/D3 receptor binding in patients with juvenile myoclonic epilepsy. Epilepsia 51(9):1699–1706. https://doi.org/10.1111/j.1528-1167.2010.02569.x
Depaulis A, Moshé SL (2002) Editorial the basal ganglia and epilepsies: translating experimental concepts to new therapies. Epileptic Disord 4(Suppl 3):S7-8
Slaght SJ, Paz T, Mahon S, Maurice N, Charpier S, Deniau JM (2002) Functional organisation of the circuits connecting the cerebral cortex and the basal ganglia: implications for the role of the basal ganglia in epilepsy. Epileptic Disord 4(Suppl 3):S9-22
Berg D, Godau J, Walter U (2008) Transcranial sonography in movement disorders. Lancet Neurol 7(11):1044–1055. https://doi.org/10.1016/S1474-4422(08)70239-4
Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K (1995) Degeneration of substantia nigra in chronic parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 45(1):182–184. https://doi.org/10.1212/wnl.45.1.182
Behnke S, Berg D, Naumann M, Becker G (2005) Differentiation of parkinson’s disease and atypical parkinsonian syndromes by transcranial ultrasound. J Neurol Neurosurg Psychiatry 76(3):423–425. https://doi.org/10.1136/jnnp.2004.049221
Berg D (2011) Hyperechogenicity of the substantia nigra: pitfalls in assessment and specificity for parkinson’s disease. J Neural Transm (Vienna) 118(3):453–461. https://doi.org/10.1007/s00702-010-0469-5
Mijajlović M, Petrović I, Stojković T, Svetel M, Stefanova E, Kostić VS (2008) [Transcranial parenchymal sonography in the diagnosis of parkinson’s disease]. Vojnosanit Pregl 65(8):601–605. https://doi.org/10.2298/vsp0808601m. (Serbian)
Mijajlovic MD (2010) Transcranial sonography in depression. Int Rev Neurobiol 90:259–272. https://doi.org/10.1016/S0074-7742(10)90018-4
Krogias C, Walter U (2016) Transcranial sonography findings in depression in association with psychiatric and neurologic diseases: a review. J Neuroimaging 26(3):257–263. https://doi.org/10.1111/jon.12328
Şenel B, Özel-Kızıl ET, Sorgun MH, Tezcan-Aydemir S, Kırıcı S (2020) Transcranial sonography imaging of brainstem raphe, substantia nigra and cerebral ventricles in patients with geriatric depression. Int J Geriatr Psychiatry 35(7):702–711. https://doi.org/10.1002/gps.5287
Walter U, Krolikowski K, Tarnacka B, Benecke R, Czlonkowska A, Dressler D (2005) Sonographic detection of basal ganglia lesions in asymptomatic and symptomatic Wilson disease. Neurology 64(10):1726–1732. https://doi.org/10.1212/01.WNL.0000161847.46465.B9
Svetel M, Mijajlović M, Tomić A, Kresojević N, Pekmezović T, Kostić VS (2012) Transcranial sonography in Wilson’s disease. Parkinsonism Relat Disord 18(3):234–238. https://doi.org/10.1016/j.parkreldis.2011.10.007
Mijajlović M, Dragasević N, Stefanova E, Petrović I, Svetel M, Kostić VS (2008) Transcranial sonography in spinocerebellar ataxia type 2. J Neurol 255(8):1164–1167. https://doi.org/10.1007/s00415-008-0862-2
Lezak MD, Howieson DB, Bigler ED, Tranel D (2012) Neuropsychological Assessment, 5th edn. Oxford University Press
Tombaugh TN (2004) Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 19(2):203–214. https://doi.org/10.1016/S0887-6177(03)00039-8
Golden CJ (2004) The Adult Luria-Nebraska Neuropsychological Battery. In: Goldstein G, Beers SR, Hersen M (eds) Comprehensive handbook of psychological assessment, intellectual and neuropsychological assessment, vol 1. John Wiley & Sons Inc, Hoboken, NJ, US, pp 133–146
Walter U, Školoudík D (2004) Transcranial sonography (TCS) of brain parenchyma in movement disorders: quality standards, diagnostic applications and novel technologies. Ultraschall Med 35(4):322–331. https://doi.org/10.1055/s-0033-1356415
Godau J, Wevers AK, Gaenslen A, Di Santo A, Liepelt I, Gasser T, Berg D (2008) Sonographic abnormalities of brainstem structures in restless legs syndrome. Sleep Med 9(7):782–789. https://doi.org/10.1016/j.sleep.2007.09.001
Raatikainen M, Kälviäinen R, Jutila L, Äikiä M (2020) Cognitive functioning in new-onset juvenile myoclonic epilepsy. Epilepsy Behav 106:107015. https://doi.org/10.1016/j.yebeh.2020.107015
Camfield CS, Striano P, Camfield PR (2013) Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav 28(Suppl 1):S15–S17. https://doi.org/10.1016/j.yebeh.2012.06.024
Jovic N (2012) Frontal lobe dysfunctions in patients with juvenile myoclonic epilepsy. J Ped Epilepsy 2:77–85
Vuong J, Devergnas A (2018) The role of the basal ganglia in the control of seizure. J Neural Transm (Vienna) 125(3):531–545. https://doi.org/10.1007/s00702-017-1768-x
Kim JH, Kim JB, Suh S, Kim DW (2017) Subcortical grey matter changes in juvenile myoclonic epilepsy. Neuroimage Clin 17:397–404. https://doi.org/10.1016/j.nicl.2017.11.001
Trinka E, Kienpointner G, Unterberger I, Luef G, Bauer G, Doering LB, Doering S (2006) Psychiatric comorbidity in juvenile myoclonic epilepsy. Epilepsia 47(12):2086–2091. https://doi.org/10.1111/j.1528-1167.2006.00828.x
Filho GM, Rosa VP, Lin K, Caboclo LO, Sakamoto AC, Yacubian EM (2008) Psychiatric comorbidity in epilepsy: a study comparing patients with mesial temporal sclerosis and juvenile myoclonic epilepsy. Epilepsy Behav 13(1):196–201. https://doi.org/10.1016/j.yebeh.2008.01.008
Iadarola MJ, Gale K (1982) Substantia nigra: site of anticonvulsant activity mediated by g-aminobutyric acid. Science 218(4578):1237–1240. https://doi.org/10.1126/science.7146907
Velísková J, Moshé SL (2006) Update on the role of substantia nigra pars reticulata in the regulation of seizures. Epilepsy Curr 6(3):83–87. https://doi.org/10.1111/j.1535-7511.2006.00106.x
Albala BJ, Moshé SL, Okada R (1984) Kainic-acid-induced seizures: a developmental study. Dev Brain Res 315(1):139–148. https://doi.org/10.1016/0165-3806(84)90085-3
Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R (1981) Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience 6(7):1361–1391. https://doi.org/10.1016/0306-4522(81)90193-7
Chen B, Xu C, Wang Y, Lin W, Wang Y, Chen L, Cheng H, Xu L, Hu T, Zhao J, Dong P, Guo Y, Zhang S, Wang S, Zhou Y, Hu W, Duan S, Chen Z (2020) A disinhibitory nigra-parafascicular pathway amplifies seizure in temporal lobe epilepsy. Nat Commun 11(1):923. https://doi.org/10.1038/s41467-020-14648-8
Velísková J, Miller AM, Nunes ML, Brown LL (2005) Regional neural activity within the substantia nigra during peri-ictal flurothyl generalized seizure stages. Neurobiol Dis 20(3):752–759. https://doi.org/10.1016/j.nbd.2005.05.007
Sperber EF, Wurpel JND, Zhao DY, Moshé SL (1989) Evidence for the involvement of nigral GABAa receptors in seizures of adult rats. Brain Res 480(1–2):378–382. https://doi.org/10.1016/0006-8993(89)90211-4
Ciumas C, Wahlin TB, Jucaite A, Lindstrom P, Halldin C, Savic I (2008) Reduced dopamine transporter binding in patients with juvenile myoclonic epilepsy. Neurology 71(11):788–794. https://doi.org/10.1212/01.wnl.0000316120.70504.d5
Habas C, Guillevin R, Abanou A (2010) In vivo structural and functional imaging of the human rubral and inferior olivary nuclei: a mini-review. Cerebellum 9(2):167–173. https://doi.org/10.1007/s12311-009-0145-1
Lefebvre V, Josien E, Pasquier F, Steinling M, Petit H (1993) Infarctus du noyau rouge et diaschisis cérébelleux croisé. Rev Neurol (Paris) 149(4):294–296 (French)
Kalnin AJ, Fastenau PS, deGrauw TJ, Musick BS, Perkins SM, Johnson CS, Mathews VP, Egelhoff JC, Dunn DW, Austin JK (2008) Magnetic resonance imaging findings in children with a first recognized seizure. Pediatr Neurol 39(6):404–414. https://doi.org/10.1016/j.pediatrneurol.2008.08.008
Jackson DC, Irwin W, Dabbs K, Lin JJ, Jones JE, Hsu DA, Stafstrom CE, Seidenberg M, Hermann BP (2011) Ventricular enlargement in new-onset pediatric epilepsies. Epilepsia 52(12):2225–2232. https://doi.org/10.1111/j.1528-1167.2011.03323.x
Acknowledgements
We are deeply grateful to our late Professor Nebojša Jović for his commitment, help and support in this work.
Funding
This work was granted by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia: 451-03-66/2024-03/200110.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by IDJ, SDJ, AK, DV, NIR, and MM. The first draft of the manuscript was written by IDJ and MŽ, MŽ and MM prepared figure 1 and all authors have commented on previous versions of the manuscript. The whole research was conceptualized and supervised by MM. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethic Committee of the Clinic for Neurology and Psychiatry for Children and Youth in Belgrade (CNPCY 11/I-15).
Patient consent
Written informed consent was obtained from all participants or their parents/caregivers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Djordjević, I., Djordjević, S., Kosać, A. et al. Transcranial brain parenchyma sonography in patients with juvenile myoclonic epilepsy. Acta Neurol Belg (2024). https://doi.org/10.1007/s13760-024-02561-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13760-024-02561-6