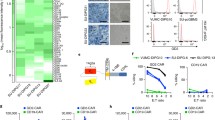
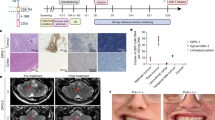
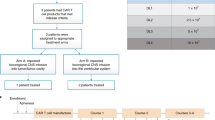
Abstract
Pediatric brain tumors are the primary cause of death in children with cancer. Diffuse midline glioma (DMG) and diffuse intrinsic pontine glioma (DIPG) are frequently unresectable due to their difficult access location, and 5-year survival remains less than 20%. Despite significant advances in tumor biology and genetics, treatment options remain limited and ineffective. Immunotherapy using T cells with a chimeric antigen receptor (CAR) that has been genetically engineered is quickly emerging as a new treatment option for these patients. High levels of expression were detected for both disialoganglioside (GD2) and B7-H3 in pediatric DMG/DIPG. Numerous studies have been conducted in recent years employing various generations of GD2-CAR T cells. The two most prevalent adverse effects found with this therapy are cytokine release syndrome, which varies in severity from mild constitutional symptoms to a high-grade disease associated with potentially fatal multi-organ failure, and neurotoxicity, known as CAR T-cell-related encephalopathy syndrome. During the acute phase of anticancer action, peri-tumoral neuro-inflammation might cause deadly hydrocephalus. The initial results of clinical trials show that the outcomes are not highly encouraging as B cell malignancies and myelomas. In vivo research on CAR T-cell therapy for DIPG has yielded encouraging results, but in human trials, the early results have shown potentially fatal side effects and very modest, but fleeting improvements. Solid tumors present a hindrance to CAR T-cell therapy because of the antigenic dilemma and the strong immune-suppressing tumor microenvironment.



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Data availability
Not applicable.
References
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C et al (2017) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 19(suppl_5):v1-88. https://doi.org/10.1093/neuonc/nox158
Das AK, Mani SK, Singh SK, Kumar S (2023) Management and outcome of unusual pediatric brain tumors: challenges experienced at a tertiary care center of a developing country. Childs Nerv Syst 39(1):169–183. https://doi.org/10.1007/s00381-022-05694-2
El-Khouly FE, Veldhuijzen van Zanten SEM, Santa-Maria Lopez V, Hendrikse NH, Kaspers GJL, Loizos G et al (2019) Diagnostics and treatment of diffuse intrinsic pontine glioma: where do we stand? J Neurooncol 145(1):177–84. https://doi.org/10.1007/s11060-019-03287-9
Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR et al (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32(4):520-537.e5. https://doi.org/10.1016/j.ccell.2017.08.017
Kieran MW, Hargrave DR, Cohen KJ, Aerts I, Dunkel IJ, Hummel TR et al (2015) Phase 1 study of dabrafenib in pediatric patients (pts) with relapsed or refractory BRAF V600E high-and low-grade gliomas (HGG, LGG), Langerhans cell histiocytosis (LCH), and other solid tumors (OST). JCO 33:10004–10004
Antonucci L, Canciani G, Mastronuzzi A, Carai A, Del Baldo G, Del Bufalo F (2022) CAR-T therapy for pediatric high-grade gliomas: peculiarities, current investigations and future strategies. Front Immunol 13:867154. https://doi.org/10.3389/fimmu.2022.867154
de Billy E, Pellegrino M, Orlando D, Pericoli G, Ferretti R, Businaro P et al (2022) Dual IGF1R/IR inhibitors in combination with GD2-CAR T-cells display a potent anti-tumor activity in diffuse midline glioma H3K27M-mutant. Neuro Oncol 24(7):1150–1163. https://doi.org/10.1093/neuonc/noab300
Leszczynska KB, Jayaprakash C, Kaminska B, Mieczkowski J (2021) Emerging advances in combinatorial treatments of epigenetically altered pediatric high-grade H3K27M gliomas. Front Genet. https://doi.org/10.3389/fgene.2021.742561
Merli P, Algeri M, Del Bufalo F, Locatelli F (2019) Hematopoietic stem cell transplantation in pediatric acute lymphoblastic leukemia. Curr Hematol Malig Rep 14(2):94–105. https://doi.org/10.1007/s11899-019-00502-2
Hong M, Clubb JD, Chen YY (2020) Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 38(4):473–488. https://doi.org/10.1016/j.ccell.2020.07.005
Haydar D, Houke H, Chiang J, Yi Z, Odé Z, Caldwell K et al (2021) Cell-surface antigen profiling of pediatric brain tumors: B7–H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery. Neuro Oncol 23(6):999–1011. https://doi.org/10.1093/neuonc/noaa278
Nazha B, Inal C, Owonikoko TK (2020) Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol 10:1000. https://doi.org/10.3389/fonc.2020.01000
Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B et al (2017) CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther 25(9):2214–2224. https://doi.org/10.1016/j.ymthe.2017.05.012
Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S et al (2018) Potent antitumor efficacy of anti-GD2 CAR T cells in H3–K27M+ diffuse midline gliomas. Nat Med 24(5):572–579. https://doi.org/10.1038/s41591-018-0006-x
Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM et al (2022) GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603(7903):934–941. https://doi.org/10.1038/s41586-022-04489-4
Ma J, Chen CC, Li M (2021) Macrophages/microglia in the glioblastoma tumor microenvironment. Int J Mol Sci 22(11):5775. https://doi.org/10.3390/ijms22115775
Dello Russo C, Lisi L, Tentori L, Navarra P, Graziani G, Combs CK (2017) Exploiting microglial functions for the treatment of glioblastoma. Curr Cancer Drug Targets 17(3):267–281. https://doi.org/10.2174/1568009616666160813191240
Lin GL, Nagaraja S, Filbin MG, Suvà ML, Vogel H, Monje M (2018) Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol Commun. https://doi.org/10.1186/s40478-018-0553-x
Grégoire H, Roncali L, Rousseau A, Chérel M, Delneste Y, Jeannin P et al (2020) Targeting tumor associated macrophages to overcome conventional treatment resistance in glioblastoma. Front Pharmacol 11:368. https://doi.org/10.3389/fphar.2020.00368
Engler JR, Robinson AE, Smirnov I, Hodgson JG, Berger MS, Gupta N et al (2012) Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PLoS ONE 7(8):e43339. https://doi.org/10.1371/journal.pone.0043339
Donovan LK, Delaidelli A, Joseph SK, Bielamowicz K, Fousek K, Holgado BL et al (2020) Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med 26(5):720–731. https://doi.org/10.1038/s41591-020-0827-2
Turk OM, Woodall RC, Gutova M, Brown CE, Rockne RC, Munson JM (2021) Delivery strategies for cell-based therapies in the brain: overcoming multiple barriers. Drug Deliv Transl Res 11(6):2448–2467. https://doi.org/10.1007/s13346-021-01079-1
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M et al (2014) Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124(2):188–195. https://doi.org/10.1182/blood-2014-05-552729
Brudno JN, Kochenderfer JN (2016) Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127:3321–3330
Maude SL, Barrett D, Teachey DT (2014) Grupp SA managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 20:119–122
Hu Y, Sun J, Wu Z, Yu J, Cui Q, Pu C et al (2016) Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J Hematol Oncol. https://doi.org/10.1186/s13045-016-0299-5
Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G et al (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 14(11):1264–1270. https://doi.org/10.1038/nm.1882
Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD et al (2011) Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118(23):6050–6056. https://doi.org/10.1182/blood-2011-05-354449
Perez Horta Z, Goldberg JL, Sondel PM (2016) Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy. Immunotherapy 8(9):1097–1117. https://doi.org/10.2217/imt-2016-0021
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL et al (2018) Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol 15(1):47–62. https://doi.org/10.1038/nrclinonc.2017.148
Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF et al (2018) Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther 26(7):1855–1866. https://doi.org/10.1016/j.ymthe.2018.05.003
Yin Y, Boesteanu AC, Binder ZA, Xu C, Reid RA, Rodriguez JL et al (2018) Checkpoint blockade reverses anergy in IL-13Rα2 humanized scFv-based CAR T cells to treat murine and canine gliomas. Mol Ther Oncolytics 11:20–38. https://doi.org/10.1016/j.omto.2018.08.002
Sterner RC, Sterner RM (2021) CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 11(4):69. https://doi.org/10.1038/s41408-021-00459-7
Chmielewski M, Abken H (2015) TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther 15(8):1145–1154. https://doi.org/10.1517/14712598.2015.1046430
Avanzi MP, Yeku O, Li X, Wijewarnasuriya DP, van Leeuwen DG, Cheung K et al (2018) Engineered tumor-targeted T cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep 23(7):2130–2141. https://doi.org/10.1016/j.celrep.2018.04.051
Hu B, Ren J, Luo Y, Keith B, Young RM, Scholler J et al (2017) Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep 20(13):3025–3033. https://doi.org/10.1016/j.celrep.2017.09.002
Hurton LV, Singh H, Najjar AM, Switzer KC, Mi T, Maiti S et al (2016) Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci USA 113(48):E7788–E7797. https://doi.org/10.1073/pnas.1610544113
Zimmermann K, Kuehle J, Dragon AC, Galla M, Kloth C, Rudek LS et al (2020) Design and characterization of an “all-in-one” Lentiviral vector system combining constitutive anti-GD2 CAR expression and inducible cytokines. Cancers (Basel) 12(2):375. https://doi.org/10.3390/cancers12020375
Krenciute G, Prinzing BL, Yi Z, Wu M-F, Liu H, Dotti G et al (2017) Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol Res 5(7):571–581. https://doi.org/10.1158/2326-6066.CIR-16-0376
Nicolas-Boluda A, Donnadieu E (2019) Obstacles to T cell migration in the tumor microenvironment. Comp Immunol Microbiol Infect Dis 63:22–30. https://doi.org/10.1016/j.cimid.2018.12.006
Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M et al (2019) CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun 10(1):4016. https://doi.org/10.1038/s41467-019-11869-4
Theruvath J, Sotillo E, Mount CW, Graef CM, Delaidelli A, Heitzeneder S et al (2020) Locoregionally administered B7–H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med 26(5):712–719. https://doi.org/10.1038/s41591-020-0821-8
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A et al (2016) Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 375(26):2561–2569. https://doi.org/10.1056/NEJMoa1610497
Abbott NJ (2013) Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36(3):437–449. https://doi.org/10.1007/s10545-013-9608-0
Das AK, Singh SK, Bhavana K, Kumar S (2023) Posterior fossa giant adenoid cystic carcinoma with skull base invasion mimicking glomus jugulare: a case report and review of literature. Rare Tumors 15:203636132211502. https://doi.org/10.1177/20363613221150218
Arvanitis CD, Ferraro GB, Jain RK (2020) The blood-brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer 20(1):26–41. https://doi.org/10.1038/s41568-019-0205-x
Akhavan D, Alizadeh D, Wang D, Weist MR, Shepphird JK, Brown CE (2019) CAR T cells for brain tumors: lessons learned and road ahead. Immunol Rev 290(1):60–84. https://doi.org/10.1111/imr.12773
Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M et al (2015) 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21(6):581–590. https://doi.org/10.1038/nm.3838
Thomas S, Straathof K, Himoudi N, Anderson J, Pule M (2016) An optimized GD2-targeting Retroviral cassette for more potent and safer cellular therapy of neuroblastoma and other cancers. PLoS ONE 11(3):e0152196. https://doi.org/10.1371/journal.pone.0152196
Long AH, Highfill SL, Cui Y, Smith JP, Walker AJ, Ramakrishna S et al (2016) Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol Res 4(10):869–880. https://doi.org/10.1158/2326-6066.CIR-15-0230
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX et al (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363(14):1324–1334. https://doi.org/10.1056/NEJMoa0911123
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517. https://doi.org/10.1056/nejmoa1407222
Kochenderfer JN, Dudley ME, Kassim SH, Somerville RPT, Carpenter RO, Stetler-Stevenson M et al (2015) Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 33(6):540–549. https://doi.org/10.1200/JCO.2014.56.2025
Turtle CJ, Hanafi L-A, Berger C, Hudecek M, Pender B, Robinson E et al (2016) Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8(355):355ra116. https://doi.org/10.1126/scitranslmed.aaf8621
Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM et al (2017) Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther 25(1):285–295. https://doi.org/10.1016/j.ymthe.2016.10.020
Neelapu SS, Locke FL, Bartlett NL, Lekakis L, Miklos D, Jacobson CA, et al (2016) Kte-C19 (anti-CD19 CAR T cells) induces complete remissions in patients with refractory diffuse large B-cell lymphoma (DLBCL): Results from the pivotal phase 2 Zuma-1. Blood 128(22):LBA-6-LBA-6. https://doi.org/10.1182/blood.v128.22.lba-6.lba-6
Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N et al (2016) Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 6(6):664–679. https://doi.org/10.1158/2159-8290.CD-16-0040
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al (2015) T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385(9967):517–528. https://doi.org/10.1016/S0140-6736(14)61403-3
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al (2013) Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med 368(16):1509–1518. https://doi.org/10.1056/nejmoa1215134
Nih.gov. [cited 2023 Apr 4]. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06
Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ et al (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358(9):877–887. https://doi.org/10.1056/NEJMoa067373
GD2 CAR T cells in diffuse intrinsic pontine gliomas (DIPG) & spinal diffuse midline glioma (DMG). Clinicaltrials.gov. [cited 2023 Apr 4]. https://clinicaltrials.gov/ct2/show/study/NCT04196413
Kernel Networks Inc. C7R-GD2.CAR T cells for patients with GD2-expressing brain tumors (GAIL-B). Case Medical Research. 2019 [cited 2023 Apr 4]; https://clinicaltrials.gov/ct2/show/study/NCT04099797
T cells expressing HER2-specific chimeric antigen receptors (CAR) for patients with HER2-positive CNS tumors—full text view—Clinicaltrials.gov. Clinicaltrials.gov. [cited 2023 Apr 4]. https://clinicaltrials.gov/ct2/show/study/NCT02442297
HER2-specific CAR T Cell Locoregional Immunotherapy for HER2-positive Recurrent/Refractory Pediatric CNS Tumors. Clinicaltrials.gov. [cited 2023 Apr 4]. https://clinicaltrials.gov/ct2/show/study/NCT03500991
Case Medical Research. Study of B7-H3-specific CAR T cell locoregional immunotherapy for diffuse intrinsic pontine glioma/diffuse Midline glioma and recurrent or refractory pediatric central nervous system tumors. Case Medical Research. 2019 [cited 2023 Apr 4]. https://clinicaltrials.gov/ct2/show/study/NCT04185038
B7-H3-specific chimeric antigen receptor autologous T-Cell therapy for pediatric patients with solid tumors (3CAR). Clinicaltrials.gov. [cited 2023 Apr 4]. https://clinicaltrials.gov/ct2/show/study/NCT04897321
Genetically modified T-cells in treating patients with recurrent or refractory malignant glioma. Clinicaltrials.gov. [cited 2023 Apr 4]. https://clinicaltrials.gov/ct2/show/study/NCT02208362
EGFR806-specific CAR T Cell Locoregional Immunotherapy for EGFR-positive Recurrent or Refractory Pediatric CNS Tumors. Clinicaltrials.gov. [cited 2023 Apr 4]. https://clinicaltrials.gov/ct2/show/study/NCT03638167
Acknowledgements
The authors acknowledge all professors and consultants in the Department of Neurosurgery for their guidance and assistance.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Anand Kumar Das and Saraj Kumar Singh contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Anand Kumar Das, Mainak Sinha, Saraj Kumar Singh, Anurag Chaudhary, Ashim Kumar Boro, Manish Agrawal, Sona Bhardwaj, Simmi Kishore, and Katyayani Kumari. The first draft of the manuscript was written by Anand Kumar Das and Mainak Sinha. Both contributed equally to the work*. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
Not required.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, A.K., Sinha, M., Singh, S.K. et al. CAR T-cell therapy: a potential treatment strategy for pediatric midline gliomas. Acta Neurol Belg (2024). https://doi.org/10.1007/s13760-024-02519-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13760-024-02519-8