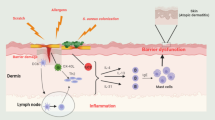
Abstract
Purpose of Review
This review focused on the mechanisms of action, safety profiles, and efficacy of new and promising systemic immunological treatments for AD. In particular, it has been highlighted how new treatments’ targets participate in the pathogenic mechanisms of AD. The introduction of new pharmacological agents can provide new therapeutic options, but there is the need to evaluate how “narrow-acting” agents like individual interleukin inhibitors will perform under the safety and efficacy profiles compared with “broad-acting” agents like JAK inhibitors. Furthermore, the article aimed to describe and compare the marketing of these medicines in the European and Latin American markets, comparing the access requirements to these innovative treatments for patients affected from atopic dermatitis in both regions.
Recent Findings
Moderate to severe AD is often refractory to first-line topical treatments, while systemic immunosuppressants have shown efficacy but have significant side effects. The limited availability of basic treatments has led to the development of targeted topical and systemic immunotherapies, including small molecule drugs and biologics, representing a new era of therapeutic innovation. In 2016, the US Food and Drug Administration (FDA) approved a topical phosphodiesterase-4 inhibitor (PDE4i), crisaborole, and in 2017, a monoclonal antibody, dupilumab.
Summary
Atopic dermatitis (AD) is a common inflammatory skin disease characterized by itching and skin barrier dysfunction. Moderate to severe AD is often refractory to first-line topical treatments, and systemic immunosuppressants have been shown to be effective but have significant adverse effects. The paucity of basic treatments has contributed to the development of targeted topical and systemic immunotherapies based on the use of small molecules and biologic drugs which can directly interact with AD pathogenetic pathways. They represent a new era of therapeutic innovation. Additional new treatments are desirable since AD is a heterogeneous disease marked by different immunological phenotypes. This manuscript will review the mechanism of action, safety profile, and efficacy of promising new systemic immunological treatments for AD which are placed on the European and Latin American markets, making a comparison between the two current regulatory frameworks. Since moderate to severe AD can result in poor quality of life, the development of targeted and well-tolerated immunomodulators is a crucial purpose in both regions. The introduction of new pharmacological agents may offer new therapeutic options.

Similar content being viewed by others
Availability of Data and Materials
Full availability of data and materials. All stated data can be provided on request to the reader.
Code Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Furue K, Ito T, Tsuji G, Ulzii D, Vu YH, Kido-Nakahara M, et al. The IL-13-OVOL1-FLG axis in atopic dermatitis. Immunology. 2019;158:281–6.
Suarez-Farinas M, Ungar B, da Rosa CJ, Ewald DA, Rozenblit M, Gonzalez J, et al. RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. J Allergy Clin Immunol. 2015;135:1218–27.
Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1–11.
Silverberg JI, Kantor R. The role of interleukins 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol Clin. 2017;35:327–34.
Bissonnette R, Pavel AB, Diaz A, Werth JL, Zang C, Vranic I, et al. Crisaborole and atopic dermatitis skin biomarkers: an intrapatient randomized trial. J Allergy Clin Immunol. 2019;144:1274–89.
Newsom M, Bashyam AM, Balogh EA, Feldman SR, Strowd LC. New and emerging systemic treatments for atopic dermatitis. Drugs. 2020;80:1041–52.
Harb H, Chatila TA. Mechanisms of dupilumab. Clin Exp Allergy. 2019;50:5–14.
Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143:135–41.
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. SOLO 1 and SOLO 2 investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–48.
Worm M, Simpson EL, Thaçi D, Bissonnette R, Lacour JP, Beissert S, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:131–43.
Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–303.
de Bruin-Weller M, Thaçi D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178:1083–101.
Beck LA, Thaçi D, Deleuran M, Blauvelt A, Bissonnette R, de Bruin-Weller M, et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2020;21:567–77.
Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44–56.
Cork MJ, Thaçi D, Eichenfield LF, Arkwright PD, Sun X, Chen Z, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥6 to <12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857–70.
Paller AS, Wollenberg A, Siegfried E, Thaçi D, Cork MJ, Arkwright PD, et al. Laboratory safety of dupilumab in patients aged 6–11 years with severe atopic dermatitis: results from a phase iii clinical trial. Paediatr Drugs. 2021;23:515–27.
•• Patruno C, Fabbrocini G, Longo G, Argenziano G, Ferrucci SM, Stingeni L, et al. Effectiveness and safety of long-term dupilumab treatment in elderly patients with atopic dermatitis: a multicenter real-life observational study. Am J Clin Dermatol. 2021;22:581–6. The objective of this study was to assess the effectiveness and safety of dupilumab in treating elderly patients with atopic dermatitis from baseline to 52 weeks. - In this study, dupilumab is an effective and safe treatment for the long-term management of atopic dermatitis in patients aged over 65 years.
Patruno C, Napolitano M, Argenziano G, Peris K, Ortoncelli M, Girolomoni G, et al. DADE - Dupilumab for Atopic Dermatitis of the Elderly study group. Dupilumab therapy of atopic dermatitis of the elderly: a multicentre, real-life study. J Eur Acad Dermatol Venereol. 2021;35:958–64.
Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, et al. ECZTRA 1 and ECZTRA 2 study investigators. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184:437–49.
Silverberg JI, Toth D, Bieber T, Alexis AF, Elewski BE, Pink AE, et al. ECZTRA 3 study investigators. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450–63.
•• Blauvelt A, Langley RG, Lacour JP, Toth D, Laquer V, Beissert S, et al. Long-term 2 year safety and efficacy of tralokinumab in adults with moderate-to-severe atopic dermatitis: interim analysis of the ECZTEND open-label extension trial. J Am Acad Dermatol. 2022;87:815–24. To evaluate the safety and efficacy of up to 2 years tralokinumab treatment in a post hoc interim analysis. - Over 2 years, tralokinumab has been well tolerated and maintained long-term control of AD signs and symptoms.
Gutermuth J, Pink AE, Worm M, Soldbro L, Bjerregård Øland C, Weidinger S, et al. Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: a placebo-controlled, randomized, phase III clinical trial (ECZTRA 7). Br J Dermatol. 2022;186:440–52.
Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taieb A, et al. Efficacy and safety of lebrikizumab (an antiIL-13 monoclonal antibody) in adults with moderate- to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78:863-71e11.
Guttman-Yassky E, Blauvelt A, Eichenfeld LF, Paller AS, Armstrong AW, Drew J, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411–20.
Ferrara F, Vitiello A. Scientific hypothesis for treatment of COVID-19’s lung lesions by adjusting ACE/ACE2 imbalance. Cardiovasc Toxicol. 2021;21(6):498–503. https://doi.org/10.1007/s12012-021-09649-y.
Ruzicka T, Hanifn JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. XCIMA Study Group. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376:826–35.
Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. 2019;145:173–82.
Nemoto O, Furue M, Nakagawa H, Shiramoto M, Hanada R, Matsuki S, et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo- controlled study. Br J Dermatol. 2016;174:296–304.
Duhen R, Ballesteros-Merino C, Frye AK, Tran E, Shu-Ching Chang T, Koguchi Y, et al. Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells. Nat Commun. 2021;12:1047.
Guttman-Yassky EE, Pavel AB, Zhou L, Estrada YD, Zhang N, Xu H, et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;144:482-93e7.
Mogul A, Corsi K, McAuliffe L. Baricitinib: the second FDA-approved JAK inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother. 2019;53:947–53.
Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80:913-21e9.
Bauman JN, Doran AC, King-Ahmad A, Sharma R, Walker GS, Lin J, et al. The pharmacokinetics, metabolism, and clearance mechanisms of abrocitinib, a selective Janus kinase inhibitor, in humans. Drug Metab Dispos. 2022;50:1106–18.
Pfizer announces positive top-line results from Phase 3 study of investigational oral Jak1 candidate, abrocitinib (Pf-04965842), in patients aged 12 and older with moderate to severe atopic dermatitis. [Internet] https://bit.ly/301uANq [updated May 15, 2019].
Gooderham MJ, Forman SB, Bissonnette R, Beebe JS, Zhang W, Banfeld C, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371–9.
Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Freidman M, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2:23.
• Simpson EL, Papp KA, Blauvelt A, Chu CY, Hong HC, Katoh N, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol. 2022;158:404–13. In this analysis of follow-up data from 2 randomized clinical trials, longer-term treatment of adolescents and adults with moderate to severe atopic dermatitis with upadacitinib demonstrated a favorable benefit-risk profile, with sustained efficacy responses through 52 weeks.
Silverberg JI, Rubini NPM, Pires MC, Rossi AB, Zhang A, Chen Z, et al. Dupilumab treatment reduces hospitalizations in adults with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol Pract. 2022;10:1279-85.e1.
Guttman-Yassky E, Thaçi D, Pangan AL, Hong HC, Paap KA, Reich A, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877–84.
Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169–81.
Cherrez-Ojeda I, Robles-Velasco K, Thomsen SF, Ramon GD, Sánchez J, Bernstein JA, et al. Challenges in the use of the treat-to-target strategy in atopic dermatitis in Latin America: a case series review. Dermatol Ther. 2023;13(3):661–72.
Sanchez J, Cherrez-Ojeda I, Galvan C, Garcia E, Hernández-Mantilla N, Londoño Garcia A, et al. The unmet needs in atopic dermatitis control in Latin America: a multidisciplinary expert perspective. Dermatol Ther. 2021;11(5):1521–40.
Meléndez GC. Manejo de la dermatitis atópica en atención primaria. Rev Médica Costa Rica Centroamérica. 2013;70(606):337–41.
Sánchez J, Ale IS, Angles MV, Fogelbach GG, Jansen AM, Takaoka R, et al. Healthcare disparities in atopic dermatitis in Latin America: a narrative review. Dermatol Ther. 2023;13(2):399–416.
• Soares GB, Orfali RL, Averbach BL, Yosipovitch G, Aoki V. Atopic dermatitis in Latin America: considerations on epidemiology, clinical and laboratory features, ethnic/racial variations, and therapeutic management. J Clin Med. 2023;12(10):3419. Diagnosing AD is challenging due to broad clinical features, ethnoracial variations, and lack of universal diagnostic criteria. Furthermore, lack of physician training, barriers to medication access, and socioeconomic inequalities hinder effective disease management in LA.
Luger T, Romero WA, Gruben D, Smith TW, Cha A, Neary MP. Clinical and humanistic burden of atopic dermatitis in Europe: analyses of the national health and wellness survey. Dermatol Ther. 2022;12(4):949–69.
Ministerio de Salud Costa Rica. Registrelo. Consulta de productos registrados. [Internet]. [cited 2024 Jan 10]. Available from: https://registrelo.go.cr/cfmx/ms/consultasPublicas/index.cfm?reporte=1&CFID=1244864&CFTOKEN=d5f9f98a57de7f63-5DD17731-DFA6-62AC-63E98A568DE1B5EA
Radi G, Simonetti O, Rizzetto G, Diotallevi F, Molinelli E, Offidani A. Baricitinib: the first JAK inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9(11):1575.
Augustin M, Misery L, von Kobyletzski L, Mealing S, Redding M, Chuang C, et al. Systematic literature review assessing the overall costs and societal impacts of moderate-to-severe atopic dermatitis in Europe. J Eur Acad Dermatol Venereol. 2022;36(12):2316–24.
•• Cork M, McMichael A, Teng J, Valdez H, Rojo R, Chan G, et al. Impact of oral abrocitinib on signs, symptoms and quality of life among adolescents with moderate-to-severe atopic dermatitis: an analysis of patient-reported outcomes. J Eur Acad Dermatol Venereol. 2022;36(3):422–33. To evaluate the impact of abrocitinib on patient-reported signs/symptoms among adolescents with moderate-to-severe AD. - Patient-reported signs/symptoms, including reduction of sleep loss and quality of life, were substantially improved with abrocitinib monotherapy in adolescents with moderate-to-severe AD.
Damiani G, Calzavara‐Pinton P, Stingeni L, Hansel K, Cusano F. “Skin Allergy” Group of SIDeMaST, et al. Italian guidelines for therapy of atopic dermatitis—adapted from consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis). Dermatol Ther. 2019;32(6):e13121.
European Medicines Agency. Dupixent [Internet]. 2023 [cited 2024 Jan 11]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent
European Medicines Agency. Ebglyss [Internet]. 2023 [cited 2024 Jan 11]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ebglyss
European Medicines Agency. Adtralza [Internet]. 2023 [cited 2024 Jan 11]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/adtralza
Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I y II. J Eur Acad Dermatol Venereol. 2018;32(5):657–82.
Acknowledgements
The authors wish to thank Dr. Maria Valeria Ziccardi for reviewing their paper.
Author information
Authors and Affiliations
Contributions
FF: conceptualization, writing—original draft, methodology, supervision, and validation; AZ: writing—review and editing and validation; MC: supervision and validation; JM: writing—review and editing and validation; EZ: writing—original draft and validation; RL: conceptualization, writing—original draft.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no Conflict of Interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
The authors consent to the publication of the manuscript.
Disclaimer
The authors declare that the opinions expressed are of a personal nature and do not in any way commit the responsibility of the Administrations to which they belong.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferrara, F., Zovi, A., Capuozzo, M. et al. Innovative Immunotherapy for the Treatment of Atopic Dermatitis: Focus on the European and Latin American Regulatory Frameworks. Curr Derm Rep (2024). https://doi.org/10.1007/s13671-024-00423-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s13671-024-00423-1