Abstract
Topical treatment plays a crucial role in psoriasis management, with non-adherence being a major barrier to treatment success. The fixed-dose combination of calcipotriol (CAL) and betamethasone dipropionate (BDP) represents the first-line choice in topical psoriasis treatment. A CAL/BDP cream based on polyaphron dispersion (PAD) Technology has emerged as a novel formulation for a more convenient topical treatment of psoriasis. This article aims to summarize the most relevant published evidence about CAL/BDP PAD-cream and its underlying PAD Technology. The PAD Technology enables CAL and BDP stability in an aqueous cream through a multimolecular shell structure, as well as it increases the penetration of both active ingredients into the epidermis and dermis. This technology also demonstrated to increase the cosmetic acceptability and to provide the desirable sensory properties for a topical psoriasis treatment. Two phase III clinical trials have been conducted so far with CAL/BDP PAD-cream. Findings from both trials revealed high efficacy with a fast onset of action, a favourable safety and tolerability profile and convenience for CAL/BDP PAD-cream compared to CAL/BDP gel. In the trial including patients with psoriasis affecting the scalp (MC2-01-C7), results support the use of CAL/BDP PAD-cream in scalp psoriasis. An anchored matching-adjusted indirect comparison (MAIC) was conducted to compare CAL/BDP PAD-cream and CAL/BDP foam, as both products had been previously compared to CAL/BDP gel. CAL/BDP PAD-cream and CAL/BDP foam showed equivalent efficacy and quality of life at their recommended treatment duration, whereas greater treatment satisfaction for CAL/BDP PAD-cream was found after one week of treatment. Overall, the high patient acceptability and treatment satisfaction observed with CAL/BDP PAD-cream in clinical trials may lead to improved adherence and hence higher efficacy in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The fixed-dose combination of calcipotriol (CAL) and betamethasone dipropionate (BDP) is the first-line choice in topical psoriasis treatment. | |
The polyaphron dispersion (PAD) Technology enables CAL/BDP stability in an aqueous cream and boosts dermal absorption, as well as it increases patient cosmetic acceptance. | |
The CAL/BDP PAD-cream revealed increased diffusion/penetration of the actives, high efficacy, a favourable safety and tolerability profile, improved quality of life and increased treatment satisfaction for patients compared to CAL/BDP gel, with a quick onset of action. | |
An anchored matching-adjusted indirect comparison (MAIC) analysis showed greater treatment satisfaction for CAL/BDP PAD-cream compared to CAL/BDP foam after the first week of treatment. | |
The CAL/BDP PAD-cream represents a novel topical formulation for the treatment of psoriasis that may lead to improved adherence and hence better efficacy in clinical practice. |
Introduction
Topical treatment represents a keystone in the management of psoriasis, as around 70–80% of patients with mild-to-moderate disease can be successfully controlled with topical therapies [1]. Topical agents are the first-line treatments in psoriasis and they can be combined with phototherapy or systemic therapy when topical treatment alone is unlikely to adequately control psoriasis [1, 2]. There are several topical treatments for the treatment of psoriasis, which exist in a variety of formulations, including lotions, creams, ointments, gels and foams [2, 3].
The available topical therapies for psoriasis include: topical corticosteroids, available in different potencies (from mild to very potent) and widely used for inflammatory skin conditions (due to their well-known vasoconstriction, anti-inflammatory, immunosuppressive and antiproliferative effects on epidermal cell turnover), but are associated with several adverse effects when used in the long-term use; vitamin D analogues, like calcitriol, calcipotriol (CAL) or tacalcitol, which exert potent effects on keratinocyte proliferation and differentiation as well as immunomodulatory effects; calcineurin inhibitors (off-label), which are less effective than topical corticosteroids but present a better safety profile; and tazarotene (a topical retinoid), which use may be limited due to adverse effects and patient acceptability [2, 3]. More recently, two new agents based on once-daily cream formulations have been approved by the United States (US) Food and Drug Administration (FDA), tapinarof, an aryl hydrocarbon receptor (AhR) agonist [4], and roflumilast, a phosphodiesterase-4 (PDE4) inhibitor [5], increasing the topical armamentarium for the treatment of psoriasis.
The choice of a topical agent between the wide range of possibilities depends, among others, on the morphological variant, the body site of psoriasis and also the patient preference [3, 6]. The main factors which influence topical therapy are described in Fig. 1. Most important advantages of topical treatments include the availability of different topical ingredients and galenic forms, the adaptability to different anatomical areas and the possible combination with phototherapy and systemics. However, there are still some unmet needs on topical therapy. The low levels of efficacy, inconvenience and fear of possible adverse effects are linked to reduced patient satisfaction [7], which in turn may lead to patients not accepting the topical agent and non-adherence [1]. As poor adherence is mainly related to cosmetic acceptability, patient preferences and satisfaction with the drug vehicle should be considered to drive up adherence and improve clinical outcomes [7, 8].

Source: Adapted from Torsekar and Gautam (2007) [6]
Factors which influence topical therapy.
The fixed-dose combination of CAL, a vitamin D analogue, and the corticosteroid betamethasone dipropionate (BDP), is suggested by American, Canadian and European treatment recommendations as the first-line treatment in mild-to-moderate psoriasis [9]. The mechanism of action of the combination therapy with CAL and BDP is based on the complementary effects of both active ingredients on the specific molecules involved in the pathophysiology of psoriasis, resulting in an overall increased therapeutic response [10]. Formulations containing both CAL and BDP have been shown to be superior to CAL or BDP as monotherapy. The CAL/BDP fixed-dose combination also permits a once-daily regimen, which lead to increased adherence [11]. As the conventional therapeutic options with the CAL/BDP fixed-dose combination, such as ointment, gel or foam, may be perceived as sticky, greasy and inconvenient to many patients, there is a need to develop a more patient-friendly topical treatment for psoriasis with the CAL/BDP combination [9]. In this context, a novel cream formulation of the CAL/BDP fixed-dose combination based on the polyaphron dispersion (PAD) Technology has emerged as a promising topical treatment for psoriasis. This article aims to be a comprehensive review of the CAL/BDP PAD-cream, summarizing the most relevant published evidence about this product and its underlying technology.
Methods
On March 2023, a literature search through the PubMed database was conducted to identify studies on the PAD Technology and the CAL/BDP PAD-cream use for the treatment of psoriasis. For the identification of publications related to this novel formulation, the search term used was ‘‘Polyaphron Dispersion Technology’’. For the identification of publications related to the CAL/BDP PAD-cream, search terms included ‘‘calcipotriol’’ AND ‘‘betamethasone’’ AND ‘‘cream’. Relevant publications reporting CAL/BDP PAD-cream results on psoriasis were included. Irrelevant references were excluded from consideration. References previously known to the authors, as well as those cited within the included articles, were considered. Posters on the same topic presented at medical conferences were also evaluated. The included publications are depicted in Table 1.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
PAD Technology
The PAD technology is a novel topical formulation and drug delivery system developed with the purpose of combining efficacy and safety in a single product, without compromising convenience of therapy. This technology is based on polyaphrons, also known as colloidal liquid aphrons and biliquid foams, dispersed in a polar solvent. PAD formulations consist of polyaphron oil droplets (range from 1 to 50 µm) formed from an inner core of nonpolar solvent (containing the active ingredients solubilized in different oils preferably close to their saturation limits) encapsulated in an outer multimolecular shell (formed of surfactants, oil and water) [12].
The utilization of PAD Technology confers both chemical and physical stability based on the high robustness of the multimolecular shell structure. The multimolecular layer of organized surfactants, oil and water separates the inner core from the continuous disperse phase (typically water) and protects potentially unstable active molecules solubilized in the oil from hydrolytic degradation. Thus, this technology allows to mix in a water-based formulation compounds that could only be mixed through oily formulations. Moreover, as active ingredients are fully solubilized in the inner core of the PAD droplet, it enables increased penetration of active ingredients to target tissues. The enhanced penetration profile of PAD formulations has been demonstrated through in vitro Franz cell skin flux studies, in vivo penetration in skin biopsies taken from minipigs and also ocular delivery in pigmented rabbits. Moreover, local tolerability is an important safety parameter for topical formulations, where high levels of surfactants can cause skin irritation. In this regard, a key benefit of PAD formulations is the low level of surfactants compared to conventional emulsion creams and lotions (up to 30-fold lower). Favourable local tolerability and safety of a PAD formulation has been demonstrated in an 8-week dermal safety study in minipigs [12].
A recent study characterized the sensorial attributes of a formulation based on the PAD Technology but without active ingredients, the PAD-cream vehicle, and compared it with conventional formulations for psoriasis, such as an ointment and an oleogel product. The vehicle formulation based on the PAD Technology showed the desirable requirements for a topical product for the treatment of psoriasis, with an easier manipulation than oleogel and better sensory properties than ointment [13].
CAL/BDP PAD-Cream
The CAL/BDP PAD-cream is a fixed-dose combination of CAL and BDP formulated using the PAD Technology, which is indicated for the topical treatment of mild-to-moderate plaque psoriasis, including scalp psoriasis, in adults. The CAL/BDP PAD-cream should be applied to affected areas once daily for up to 8 weeks. The concentrations of active ingredients (50 µg/g CAL and 0.5 mg/g betamethasone as dipropionate, BDP) are identical to those previously marketed CAL/BDP fixed-dose combination products. However, in this case the PAD Technology allows for a fast absorbing, stable and easy-to-apply aqueous formulation of CAL and BDP, despite their pH-related instability when combined in the presence of water. Each PAD droplet contains a fully solubilized specific active, CAL or BDP, surrounded by a multimolecular shell structure, which provides high robustness against coalescence and maintain their physical–chemical stability [9, 12].
Clinical Evidence
Increased Diffusion/Penetration of CAL and BDP
Two studies, one in vitro and one in vivo, have evaluated the diffusion/penetration of CAL and BDP through the epidermis and dermis with the CAL/BDP PAD-cream as compared to CAL/BDP gel [14, 15]. In the in vitro Franz cell skin flux study, consistently higher amounts of CAL and BDP diffused through the epidermis from the CAL/BDP PAD-cream compared with the CAL/BDP gel. The CAL/BDP PAD-cream delivered significantly greater amounts of both BDP and CAL over the 72 h timescale of the experiment [14]. Consistent results were obtained in the in vivo study conducted in 10 healthy female subjects, where CAL/BDP PAD-cream delivered more BDP into the upper (stratum corneum) and lower layers (epidermis) of the skin than CAL/BDP gel [15].
Phase III Trials
Two phase III trials have been conducted with the CAL/BDP PAD-cream, one in the US (MC2-01-C2, NCT03308799) and one in Europe (MC2-01-C7, NCT03802344) [16]. Both phase III trials had an almost identical study design (Fig. 2). In the two cases, adult patients with mild-to-moderate psoriasis were randomly assigned in a 3:1:3 ratio to receive either the CAL/BDP PAD-cream, PAD-cream vehicle or CAL/BDP gel once daily for 8 weeks [16]. The eligible trial population for each phase III trial is described in Table 2. Baseline demographic and disease characteristics of patients included in the modified intention-to-treat (mITT) population are shown in Table 3.

Source: Adapted from Pinter et al. 2022a [16]
Design of the phase III trials conducted with the CAL/BDP PAD-cream. BDP betamethasone dipropionate, CAL calcipotriol, EU Europe, PAD polyaphron dispersion, R randomization, US United States, W week.
Although both studies had almost the same study design, their primary endpoints were different. In the US trial (MC2-01-C2) the primary endpoint was the proportion of patients in each treatment group with Physician Global Assessment (PGA) treatment success at Week 8, while in the European trial (MC2-01-C7) the primary endpoint was percentage change in modified Psoriasis Area and Severity Index (mPASI) from baseline at Week 8 [17, 18].
The results presented in sections below correspond to post-hoc pooled analyses of both phase III clinical trials based on the mITT population. However, only single trial results are shown for the improvement of itch (it was only assessed in the US trial, MC2-01-C2) and outcomes on scalp (patients with scalp psoriasis were only included in the European trial, MC2-01-C7). Missing data was imputed using multiple imputation for all endpoints, except for Psoriasis Treatment Convenience Scale (PTCS) for which missing data was handled through a Last Observation Carried Forward approach.
Efficacy
In the pooled analysis of both phase III trials, the PGA was evaluated through three different outcomes: (1) PGA improvement, defined as any improvement (≥ 1 grade) in PGA from baseline; (2) PGA controlled disease, defined as any improvement in PGA to score of 0 (‘clear’) or 1 (‘almost clear’) from baseline; and (3) PGA treatment success, defined as a PGA score of 0 (‘clear’) or 1 (‘almost clear’) and with a minimum 2 points improvement from baseline. Other efficacy outcomes assessed during phase III trials were the mean percentage change from baseline in mPASI score, the percentage of patients achieving mPASI75 and the percentage of patients achieving ≥ 4-points improvement of itch in a numerical rating scale (NRS) (Table 4).
PGA treatment success was observed in both phase III clinical trials [16]. A statistically significant higher proportion of patients in the CAL/BDP PAD-cream group than in the CAL/BDP gel group achieved PGA treatment success at Week 8 (43.2% vs 31.9%; P < 0.0001). The difference in PGA treatment success between CAL/BDP PAD-cream and CAL/BDP gel was statistically significant from Week 4 and onwards (Table 4). The efficacy of CAL/BDP PAD-cream, assessed through the PGA treatment success, improved as baseline severity increased, while CAL/BDP gel lost efficacy as baseline severity increased. PGA treatment success for patients with BSA ≤ 10 and BSA > 10 was 41.7% and 48.3%, respectively, while the corresponding values for CAL/BDP gel were 33.0% and 28.7% [16]. Already at Week 1, significantly more patients in the CAL/BDP PAD-cream group compared to PAD-cream vehicle group achieved PGA treatment success (3.6% vs 0.0%; P < 0.0001), PGA controlled disease (7.8% vs 1.3%; P < 0.0001) and ≥ 1-grade PGA improvement (36.0% vs 12.6%; P < 0.0001) [19].
PGA on the scalp was evaluated at the European phase III trial (MC2-01-C7), including patients with at least 10% of scalp involvement and a PGA on the scalp of at least mild severity (grade 2) at baseline (CAL/BDP cream, n = 112; PAD-cream vehicle, n = 38) [20]. At Week 8, the 50.8% of patients achieved PGA treatment success on the scalp in the CAL/BDP PAD-cream group versus 9.3% in the PAD-cream vehicle group (P = 0.0001). Comparable differentiation between the two arms was also present at Week 4 (P = 0.0095) and Week 6 (P = 0.0007) (Table 4). Statistically significant differences in PGA controlled disease on the scalp, defined as any improvement in PGA to score of 0 ('clear') or 1 ('almost clear') from baseline, between CAL/BDP PAD-cream and PAD-cream vehicle were already seen at Week 1 (29.5% vs 0.1%; P < 0.0001). Comparable differentiation between CAL/BDP PAD-cream and PAD-cream vehicle was also present at Week 6 (P < 0.0001) and Week 8 (P < 0.0001). At Week 4, 52.1% of patients in the CAL/BDP PAD-cream group achieved PGA controlled disease on the scalp, increasing to 68.3% at Week 8 (Table 4).
The mean percentage change in mPASI score from baseline to Week 8 was statistically greater for CAL/BDP PAD-cream than CAL/BDP gel (− 64.6% vs − 56.4%; P < 0.0001). The difference in mPASI reduction between both groups was statistically significant as early as Week 1 (P = 0.0009) (Table 4). The percentage of patients achieving mPASI75 was statistically significant higher in the CAL/BDP PAD-cream group than in the CAL/BDP gel group at Week 4 (22.1% vs 13.7%; P = 0.0004), with a statistically significant difference being maintained at Week 6 (P < 0.0001) and Week 8 (P = 0.0011) (Table 4). The percentage of patients with BSA ≤ 10 and > 10 obtaining mPASI75 with CAL/BDP PAD-cream was 43.6% and 46.5%, while the corresponding values for CAL/BDP gel were 35.2% and 32.5% (P = 0.0044 and P = 0.0012, respectively) [16].
The improvement of itch was evaluated in the US trial (MC2-01-C2) through a 11-point NRS scale. A 4-points or greater improvement of itch in the NRS scale was considered clinically meaningful [21]. A higher percentage of patients obtained a ≥ 4-point improvement of itch in the CAL/BDP PAD-cream group compared with CAL/BDP gel already at Week 1 (44.0% vs 36.9%; P = 0.0241). At Week 4, a numerically higher proportion of patients in the CAL/BDP PAD-cream group compared with the CAL/BDP gel group achieved a ≥ 4-point improvement of itch (60.2% vs 55.8%), but the difference was not statistically significant (Table 4). Itch was not evaluated in the European trial (MC2-01-C7).
Safety
Table 5 shows the adverse reactions reported for CAL/BDP PAD-cream during the two phase III clinical trials (MC2-01-C2 and MC2-01-C7). All adverse reactions were observed at a frequency below 1% (uncommon). The most common System Organ Class was ‘General disorders and administration site conditions’ (2.7%), with application site pain, irritation and pruritus being the most frequent local site reactions (each with a frequency of 0.7%) (Table 5). When comparing the safety profile of CAL/BDP PAD-cream with CAL/BDP gel and PAD-cream vehicle, there were no statistically significant differences between treatment groups in relation to the proportion of patients experiencing a treatment-emergent adverse event (TEAE). Adverse drug reactions were seen in 4.1%, 2.6% and 6.6% of patients in the CAL/BDP PAD-cream, CAL/BDP gel and PAD-cream vehicle groups, respectively. The adverse reactions reported for CAL/BDP PAD-cream were similar to other CAL/BDP fixed-dose combinations. A total of 26 (2.0%) patients reported a serious adverse event, none of which was assessed to be related to the trial medication by the investigator, and the frequency was similar between the three treatment groups. Moreover, laboratory data did not indicate alteration of the calcium metabolism driven by CAL in the CAL/BDP PAD-cream group nor in the other treatment groups during the phase III trials [16].
Quality of Life/Patient Reported Outcomes
The impact of CAL/BDP PAD-cream treatment on treatment satisfaction and quality of life was assessed through the following patient-reported outcomes during the phase III trials: Subject Global Assessment (SGA), Dermatology Life Quality Index (DLQI), PTCS and EuroQol-visual analogue scale (EQ-VAS) (Table 6).
The proportion of patients evaluating their treatment to have improved by 2 grades to clear or very mild disease on the 5-grade SGA scale, named as SGA success, was significantly higher in the CAL/BDP PAD-cream group compared to the CAL/BDP gel group at Week 4 (24.4% vs 16.8%; P = 0.0006), Week 6 (35.6% vs 25.1%, P < 0.0001) and Week 8 (44.2% vs 27.9%, P < 0.0001) (Table 6). After one week of treatment, a significantly higher proportion of patients in the CAL/BDP PAD-cream group achieved SGA success (8.2% vs 2.2%; P = 0.0096) and ≥ 1-grade improvement in SGA score (45.6% vs 31.3%; P = 0.0003) compared to the PAD-cream vehicle group [19].
Regarding DLQI results, the CAL/BDP PAD-cream group had a significantly greater improvement in the mean change from baseline in total DLQI score compared to the CAL/BDP gel group at Week 4 (− 5.7 vs − 5.0; P = 0.0002) and also at Week 8 (− 6.5 vs − 5.6; P < 0.0001) (Table 6). The percentage of patients achieving DLQI satisfaction, defined as a DLQI score of 0 or 1 (i.e., no impact of disease on the patient’s life), was significantly higher in the CAL/BDP PAD-cream group than in the CAL/BDP gel group at Week 1 (18.9% vs 15.6%, P = 0.0435) and Week 8 (43.8% vs 34.2%; P = 0.0005) (Table 6). A 4-points or greater improvement of DLQI was considered to be the minimal clinically important difference (MCID) [22]. A significantly higher proportion of patients achieved a ≥ 4-point DLQI improvement from baseline in the CAL/BDP PAD-cream group compared to CAL/BDP gel group at all evaluated timepoints, including Week 1 (45.2% vs 38.1%; P = 0.0028), Week 4 (61.3% vs 54.5%; P = 0.0108) and Week 8 (65.7% vs 58.8%; P = 0.0129) (Table 6). When focusing on individual DLQI items, 8 out of 10 questions were significantly in favour of CAL/BDP PAD-cream over CAL/BDP gel at Week 8, including questions addressing influence on the choice of clothing, sexual difficulties and also difficulties to do sports. For many of the individual items, the statistical difference was already seen at Week 4, and with regards to whether the treatment influences messiness at home or taking up time (as defined in DLQI item 10), the difference was found to be already significant at Week 1 in favour of CAL/BDP PAD-cream (P = 0.0034) [23].
The impact and convenience of the psoriasis treatment was evaluated by patients at Week 1, Week 4 and Week 8 through the PTCS. This scale included five self-reported, treatment-specific questions rated on a 10-point scale (range from 1 to 10) and a further sixth question regarding the patient’s overall satisfaction with the medical treatment. The PTCS Total Score is obtained by the sum of the first five questions (range from 5 to 50) [22]. The mean PTCS Total score was significantly higher for the CAL/BDP PAD-cream compared with the CAL/BDP gel already at Week 1 (39.1 vs 36.4; P < 0.0001). Similarly, significantly greater treatment convenience for CAL/BDP PAD-cream than CAL/BDP gel was also observed at Week 4 (39.6 vs 36.6; P < 0.0001) and Week 8 (40.4 vs 37.0; P < 0.0001) (Table 6). When considering individual PTCS questions, pooled data from both phase III trials also demonstrated significantly higher scores in favour of CAL/BDP PAD-cream, including questions addressing the greasiness of the formulation and the overall satisfaction with the medical treatment [22].
In the EQ-VAS, the patient provided a perception of his/her current overall health (range from 0 to 100), with higher scores corresponding to a better overall health [22]. The EQ-VAS improved in all treatment arms, with a significantly better improvement with CAL/BDP PAD-cream than with CAL/BDP gel at Week 8 (6.6 vs 6.1; P = 0.0158) (Table 6).
Comparison Versus CAL/BDP Foam
As there was no published direct comparison of the CAL/BDP foam and CAL/BDP PAD-cream, an indirect comparison was made. Bewley et al. had access to individual patient data for the two phase III trials for CAL/BDP PAD-cream (MC2-01-C2 and MC2-01-C7) and combined them with aggregated summary data of two CAL/BDP foam trials available in publications (the PSO-ABLE [NCT02132936] and PSO-INSIGHTFUL [NCT02310646] trials). These two CAL/BDP foam trials were selected as they were the only ones sharing a common comparator with the CAL/BDP PAD-cream trials, the CAL/BDP gel. The presence of this common comparator allowed for an anchored matching-adjusted indirect comparison (MAIC) to be undertaken [24].
A total of 11 outcomes were chosen to analyse efficacy, quality of life and treatment satisfaction. Outcomes were assessed at the recommended treatment duration for each treatment (Week 8 for CAL/BDP PAD-cream and CAL/BDP gel, and Week 4 for CAL/BDP foam) except treatment satisfaction that was assessed after one week for each treatment. Treatment satisfaction was measured with six equivalent questions from the PTCS of CAL/BDP PAD-cream trials and the Topical Product Usability Questionnaire (TPUQ) of the PSO-INSIGHTFUL study. The comparison of treatment satisfaction outcomes could only be made after one week of treatment because, in the PSO-INSIGHTFUL study, CAL/BDP foam and CAL/BDP gel were used only for one week, and this was the only study comparing the foam and gel formulations in these satisfaction and convenience variables [24].
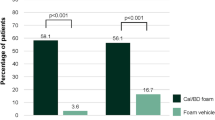
There were no statistically significant differences in PGA success, mPASI75 or DLQI outcomes between CAL/BDP PAD-cream and CAL/BDP foam when they were compared after their recommended treatment durations (8 weeks for cream and 4 weeks for foam). However, for treatment satisfaction after one week of treatment, CAL/BDP PAD-cream obtained significantly greater results than CAL/BDP foam in all domains of the questionnaires except for ‘easily incorporated into daily routine’ [24]. The satisfaction/convenience results in favour of CAL/BDP PAD-cream were confirmed in a recently published pilot trial [25].
Kircik et al. evaluated patient preference for CAL/BDP PAD-cream versus CAL/BDP foam through a pilot study involving 20 patients. Ten patients additionally had scalp psoriasis. CAL/BDP PAD-cream outperformed the foam formulation on several key measures for vehicle features and patient satisfaction. More than half of the patients preferred CAL/BDP PAD-cream over CAL/BDP foam for scalp (60%) and non-scalp application (55%) [25].
Discussion
Although topical therapy is essential in the management of psoriasis, poor patient adherence to topicals impacts its long-term effectiveness [7, 26]. Considering that an improvement in the vehicle has the potential to result in significant clinical patient benefits [27], new drug vehicles for the topical treatment of psoriasis should be designed taking into account patient preferences. In this context, the CAL/BDP PAD-cream represents a novel promising formulation based on PAD Technology that combines high efficacy, favourable safety and tolerability and patient convenience in a single product for topical psoriasis treatment [9].
The CAL/BDP PAD-cream stands out because of the novelty of its formulation, which has been designed to be cosmetically pleasing and to be applicable to several body locations, including the scalp. PAD formulations are typically oil-in-water dispersions consisting of oil droplets encapsulated in a multi-molecular shell structure that protects potentially unstable active molecules solubilized in the oil from hydrolytic degradation. Through the PAD Technology, CAL and BDP can be stable even in the presence of water, which otherwise would lead to significant degradation of the two active ingredients [12]. The PAD Technology also allows an optimal delivery of actives to the target tissue [12], which has been demonstrated through two diffusion/penetration studies [14, 15].
In the two phase III trials, CAL/BDP PAD-cream obtained good efficacy results. After 8 weeks of once-daily use, there was a greater achievement of PGA treatment success, an increased reduction of mPASI and a higher proportion of patients reaching mPASI75 in the CAL/BDP PAD-cream group than in the CAL/BDP gel [16]. CAL/BDP PAD-cream demonstrated to have also a quick onset of action, as there were statistically significant differences in favour of CAL/BDP PAD-cream already seen at Week 1 in both efficacy and patient-reported outcomes [16, 19]. Moreover, results from the European trial (MC2-01-C7) also support the use of CAL/BDP PAD-cream on the scalp. As mentioned above, the PAD Technology provides high flexibility in topical drug design and allows the application of the product to several body locations [12]. The higher efficacy of the CAL/BDP PAD-cream as compared to CAL/BDP gel obtained in the phase III trials may be explained due to the increased diffusion/penetration of actives given through the PAD Technology. Two diffusion/penetration studies have demonstrated that the CAL/BDP PAD-cream deliver higher amounts of CAL and BDP through the epidermis and dermis than CAL/BDP gel [14, 15].
Regarding safety results of the phase III trials, the CAL/BDP PAD-cream revealed a favourable safety profile, on par with the safety profile of other CAL/BDP products. There were no adverse drug reactions reported with a frequency of > 1% associated with the CAL/BDP PAD-cream, with the majority of adverse drug reactions being local site reactions [16].
Based on patient-reported outcomes results, the CAL/BDP PAD-cream seems to be more accepted by the patients than the CAL/BDP gel. The PTCS results showed superior treatment convenience for CAL/BDP PAD-cream compared with the CAL/BDP gel for all studied timepoints. Furthermore, the SGA success reported by patients mirrored the PGA treatment success reported by physicians, showing similar results at Week 8. The health-related quality of life, assessed through the DLQI, consistently demonstrated a significantly greater improvement for CAL/BDP PAD-cream than CAL/BDP gel [22]. Moreover, there is indirect evidence that patients could find CAL/BDP PAD-cream to be more convenient than CAL/BDP foam. Through an anchored MAIC analysis, a type of robust indirect comparison, CAL/BDP PAD-cream was compared to CAL/BDP foam as both products had been previously compared to CAL/BDP gel. Although CAL/BDP PAD-cream and CAL/BDP foam showed no statistical differences for efficacy and quality of life outcomes, there was a statistically significant effect in favour of CAL/BDP PAD-cream in all domains except one of the treatment satisfaction assessments after one week of treatment [24]. These results are in line with a recently published study including 20 patients, in which patients showed high levels of satisfaction with the CAL/BDP PAD-cream and preference over the CAL/BDP foam for the treatment of body and scalp psoriasis [25]. The good convenience and treatment satisfaction obtained with CAL/BDP PAD-cream may be explained by its good sensory attributes. In a sensorial analysis, the CAL/BDP PAD-cream vehicle, which had the exact same properties as CAL/BDP PAD-cream, showed low stickiness, low greasiness, good spreadability and once the CAL/BDP PAD-cream vehicle was absorbed, the gloss disappeared quickly, leaving low stickiness and a low amount of residue [13]. These attributes correspond to the ones generally preferred by patients for topical psoriasis treatments [28]. This is particularly relevant in psoriasis clinical practice, because if patients are satisfied with the treatment, they will be willing to continue using the treatment, which will lead to increased patient adherence and hence better efficacy.
Taking everything into account, the CAL/BDP PAD-cream represents a novel topical formulation for psoriasis treatment based on PAD Technology. Further investigations should take place, including head-to-head clinical trials versus CAL/BDP foam to confirm the results obtained in the MAIC analysis, as well as real-world evidence of CAL/BDP PAD-cream to support the results of phase III trials and expected increased adherence in a real-world setting.
Conclusion
The CAL/BDP PAD-cream is a novel formulation containing the fixed-dose combination of CAL/BDP, designed for a convenient topical treatment of plaque psoriasis of the body and scalp, in adults. The PAD Technology enables CAL/BDP stability in aqueous cream and boosts dermal absorption, as well as it increases patient cosmetic acceptance. Overall, the high patient acceptability and the treatment satisfaction of CAL/BDP PAD-cream shown in clinical trials may lead to improved patient adherence and, consequently, enhanced treatment outcomes in clinical practice, making this novel formulation based on PAD Technology a very interesting topical treatment for psoriasis.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. All data presented in this article is based on previously published results.
References
Pinter A, van de Kerkhof P. The role of topical therapies along the psoriasis patient journey: an overview from the Symposium “Tailoring topical psoriasis treatments to patients” needs and expectations’ of the 30th EADV Congress 2021. J Eur Acad Dermatol Venereol. 2023;37(Suppl 1):3–8.
Chat VS, Kearns DG, Uppal SK, Han G, Wu JJ. Management of psoriasis with topicals: applying the 2020 AAD-NPF guidelines of care to clinical practice. Cutis. 2022;110(2 Suppl):8–14.
Le Roux E, Frow H. Diagnosis and management of mild to moderate psoriasis. Prescriber. 2020;31(7–8):9–17.
Nogueira S, Rodrigues MA, Vender R, Torres T. Tapinarof for the treatment of psoriasis. Dermatol Ther. 2022;35(12): e15931.
Lé AM, Torres T. New topical therapies for psoriasis. Am J Clin Dermatol. 2022;23(1):13–24.
Torsekar R, Gautam MM. Topical therapies in psoriasis. Indian Dermatol Online J. 2017;8(4):235–45.
Puig L, Carrascosa JM, Belinchón I, Fernández-Redondo V, Carretero G, Ruiz-Carrascosa JC, et al. Adherence and patient satisfaction with topical treatment in psoriasis, and the use, and organoleptic properties of such treatments: a Delphi study with an expert panel and members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2013;104(6):488–96.
Vasconcelos V, Teixeira A, Almeida V, Teixeira M, Ramos S, Torres T, et al. Patient preferences for attributes of topical anti-psoriatic medicines. J Dermatolog Treat. 2019;30(7):659–63.
Pinter A, Gold LS, Reich A, Green LJ, Praestegaard M, Selmer J, et al. A novel, fixed-dose calcipotriol and betamethasone dipropionate cream for the topical treatment of plaque psoriasis: Direct and indirect evidence from phase 3 trials discussed at the 30th EADV Congress 2021. J Eur Acad Dermatol Venereol. 2023;37(Suppl 1):14–9.
Segaert S, Shear NH, Chiricozzi A, Thaçi D, Carrascosa JM, Young H, et al. Optimizing anti-inflammatory and immunomodulatory effects of corticosteroid and vitamin D analogue fixed-dose combination therapy. Dermatol Ther (Heidelb). 2017;7(3):265–79.
Megna M, Cinelli E, Camela E, Fabbrocini G. Calcipotriol/betamethasone dipropionate formulations for psoriasis: an overview of the options and efficacy data. Expert Rev Clin Immunol. 2020;16(6):599–620.
Praestegaard M, Steele F, Crutchley N. Polyaphron dispersion technology, a novel topical formulation and delivery system combining drug penetration, local tolerability and convenience of application. Dermatol Ther (Heidelb). 2022;12(10):2217–31.
García N, Guiró P, Galván J, Crutchley N, Praestegaard M, Iversen L, et al. Sensory properties analysis of a calcipotriol and betamethasone dipropionate cream vehicle formulated with an innovative PAD Technology for the treatment of plaque psoriasis on the skin and scalp. Drugs Context. 2023;12:1–8.
Crutchley N, Georgiou M, Praestegaard M, Steele F. PAD TechnologyTM-based CAL/BDP cream demonstrates superior human skin flux properties compared to topical suspension/gel. Poster presented at the FC22 Dermatology Conference (United States). https://novan.com/wp-content/uploads/2022/10/FC22_EPI_Crutchley_IVRT-Flux_poster.pdf. Accessed 14 April 2023.
Draelos ZD, Draelos MM, Steele F, Georgiou M, Praestegaard M. Enhanced skin deposition of betamethasone dipropionate from a novel formulation and drug delivery technology. Dermatol Ther (Heidelb). 2023;13(8):1763–1771.
Pinter A, Green LJ, Selmer J, Praestegaard M, Gold LS, Augustin M, et al. A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis. J Eur Acad Dermatol Venereol. 2022;36(2):228–36.
ClinicalTrials.gov. NCT03802344: This trial is a randomised, multicentre, investigator-blind, vehicle and comparator-controlled, parallel-group trial With the purpose of evaluation efficacy, safety and convenience of the MC2-01 cream. https://clinicaltrials.gov/ct2/show/NCT03802344. Accessed 14 April 2023.
ClinicalTrials.gov. NCT03308799: Clinical Trial evaluating efficacy and safety of MC2-01 cream. https://clinicaltrials.gov/ct2/show/NCT03308799. Accessed 14 April 2023.
Pinter A, Iversen L, Praestegaard M, Stein Gold L. Fixed dose calcipotriene (CAL) and betamethasone dipropionate (BDP) cream significantly improves plaque psoriasis at week one in a pooled analysis of phase 3 data. Poster presented at the 31st EADV Congress 2022, 7–10 September (Milan, Italy). Abstract ID: 1738.
Pinter A, Praestegaard M, Selmer J, Reich A. Efficacy of calcipotriene and betamethasone dipropionate cream based on PAD Technology in patients with scalp psoriasis. Poster presented at the 31st EADV Congress 2022, 7–10 September (Milan, Italy). Poster number: P1528.
Stein Gold L, Green LJ, Dhawan S, Vestbjerg B, Praestegaard M, Selmer J. A phase 3, randomized trial demonstrating the improved efficacy and patient acceptability of fixed dose calcipotriene and betamethasone dipropionate cream. J Drugs Dermatol. 2021;20(4):420–5.
Armstrong A, Pinter A, Selmer J, Præstegaard M, Reich A, Koo J. Pooled analysis demonstrating superior patient- reported psoriasis treatment outcomes for calcipotriene/betamethasone dipropionate cream versus suspension/gel. J Drugs Dermatol. 2022;21(3):242–8.
Halioua B, Bewley A, Snel-Prentø A. Dans le traitement du psoriasis en plaques, la crème CAL/BDP a montré moins d’interférences avec le choix vestimentaire et d’autres activités quotidiennes du patient par rapport au gel CAL/BDP:analyse groupée de 2 essais de phase 3, randomisés, contrôlés. Poster presented at the JDP Congress 2022, 29 November–03 December.
Bewley A, Barker E, Baker H, Green W, Avey B, Pi-Blanque A, et al. An anchored matching-adjusted indirect comparison of fixed-dose combination calcipotriol and betamethasone dipropionate (Cal/BDP) cream versus Cal/BDP foam for the treatment of psoriasis. J Dermatolog Treat. 2022;33(8):3191–8.
Kircik C, Kircik L. Patient preference for calcipotriene and betamethasone dipropionate cream versus foam for the topical treatment of psoriasis: a pilot study. J Drugs Dermatol. 2023;22(3):271–3.
Bewley A, van de Kerkhof P. Engaging psoriasis patients in adherence and outcomes to topical treatments: a summary from the Symposium “Tailoring topical psoriasis treatments to patients” needs and expectations’ of the 30th EADV Congress 2021. J Eur Acad Dermatol Venereol. 2023;37(Suppl 1):9–13.
Iversen L, Dauden E, Segaert S, Freeman K, Magina S, Rigopoulos D, et al. Reformulations of well-known active ingredients in the topical treatment of psoriasis vulgaris can improve clinical outcomes for patients. J Eur Acad Dermatol Venereol. 2017;31(8):1271–84.
Svendsen MT, Feldman SR, Tiedemann SN, Sørensen ASS, Rivas CMR, Andersen KE. Psoriasis patient preferences for topical drugs: a systematic review. J Dermatolog Treat. 2021;32(5):478–83.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial assistance in the preparation of this article was provided by Paula Casajust, MSc of TFS HealthScience. Support for this assistance was funded by Almirall S.A.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Almirall S.A.
Author information
Authors and Affiliations
Contributions
Tiago Torres, Jordi Galván, Nigel Crutchley, Morten Praestegaard, Lars Iversen, Paolo Gisondi, José Manuel Carrascosa, Bruno Halioua, Anthony Bewley and Andreas Pinter contributed to the study conception and design. Literature search, data collection and analysis, and writing of the first draft was performed by Tiago Torres and Jordi Galván. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Tiago Torres has received honoraria for acting as a consultant and/or as a speaker at events sponsored by AbbVie, Almirall, Amgen, Arena Pharmaceuticals, Biocad, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Leo Pharma, MSD, Novartis, Pfizer, Samsung-Bioepis, Sandoz, and Sanofi. Jordi Galván is an employee of Almirall. Nigel Crutchley, Morten Praestegaard and Lars Iversen are employees of MC2 Therapeutics. Lars Iversen has also served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Astra Zeneca, BMS, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen Cilag, Kyowa, Leo Pharma, MSD, Novartis, Pfizer, Regranion, Samsung, Union Therapeutics, UCB. Paolo Gisondi has been a consultant and/or speaker for Abbvie, Almirall, Amgen, Janssen, Eli Lilly, Novartis, Pierre Fabre, Sanofi and UCB. José Manuel Carrascosa has received honoraria or fees as investigator, speaker, and/or advisor from Pfizer, Sanofi, Lilly, AbbVie, Leo-Pharma, Boehringer-Ingelheim and UCB. Bruno Halioua declares that he has no competing interests. Anthony Bewley has served as a consultant to Abbvie, Almirall, Celgene, Galderma, Janssen, Leo Pharma, Lilly, Novartis, Sanofi and UCB; has participated in advisory boards for Psoriasis Association, Changing Faces, ISG and NES; has received grants from EADV and travel grants from Janssen, Leo and Almirall; has participated in guidelines committees of BAD; is working as an editor for the book Practical Psychodermatology, as president for ESDaP and as a chair for APPGOS. Andreas Pinter has received honoraria as investigator and/or has received speakers’ honoraria and/or has received grants and/or has been an advisor for the following companies: AbbVie, Almirall, Amgen, Biogen Idec, Boehringer-Ingelheim, Celgene, Celltrion, GSK, Eli-Lilly, Galderma, Hexal, Janssen, LEO-Pharma, MC2, Medac, Merck Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Sanofi-Genzyme, Schering-Plough, UCB Pharma and Zuellig Pharma.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Torres, T., Galván, J., Crutchley, N. et al. Calcipotriol and Betamethasone Dipropionate Cream Based on PAD Technology for the Treatment of Plaque Psoriasis: A Narrative Review. Dermatol Ther (Heidelb) 13, 2153–2169 (2023). https://doi.org/10.1007/s13555-023-01003-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-01003-0