Abstract
Introduction
Basal cell carcinoma of the facial region remains a challenge for contemporary oncology due to the presence of aesthetic regions and critical organs. Surgery is not always the optimal solution, and high dose rate (HDR) brachytherapy has emerged as an organ-sparing treatment method whose effectiveness has been proven by a growing number of publications. Dermoscopy is a diagnostic tool that bridges clinical and pathological examination of skin lesions. It is routinely used for diagnosis, monitoring of treatment, and post-treatment evaluation; however, the literature lacks data concerning changes in dermoscopic patterns of skin cancers during and after irradiation.
Methods
Our team conducted a prospective non-randomized trial of 39 patients with high-risk basal cell carcinomas (BCCs), mostly localized within the high-risk zone (H-zone) of the facial region, and who qualified for HDR brachytherapy. HDR contact brachytherapy with custom-made surface molds was introduced, delivering a dose of 45 Gy in 9 fractions prescribed to the tumor. Every patient was observed clinically and dermoscopically at three observational points: before treatment, at the end of treatment (3rd week), and 24 weeks after the end of therapy. The evolution of clinical and dermoscopic patterns was observed by two independent dermoscopists using current diagnostic criteria. A database of 12,088 photographic observations was evaluated.
Results
Univariate logistic regression proved that brachytherapy decreases the number of clinical and dermoscopic patterns typical for basal cell carcinoma, as well as dermoscopic features not related to BCC, presumably due to the formation of scar tissue. In addition, univariate logistic regression with random effects proved a positive correlation between tumor size and presence of various dermoscopic patterns typical for BCC.
Conclusion
Dermoscopy is proven to be easy to perform and an adequate monitoring tool for patients with BCCs undergoing HDR brachytherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Searched literature lacks data regarding evolution of dermoscopic patterns among patients undergoing HDR brachytherapy. |
What have We learned from this study? |
HDR brachytherapy decreases number of clinical and dermoscopic features of basal cell carcinoma. |
Changes in clinical observation correlate with evolution of dermoscopic features. |
Dermoscopy is easy to perform and adequate monitoring tool for patients with BCC’s undergoing HDR brachytherapy. |
HDR brachytherapy is well tolerated method with good cosmetic outcome. |
Introduction
Non-melanoma skin cancers (NMSC) are the most common malignancies in the Caucasian population of the world [1,2,3]. The numbers are thought to be under evaluated due to a low reporting rate. Fair skin, sunbathing, and age are common risk factors associated with occurrence of NMSCs [4]. Basal cell carcinoma (BCC) is a predominant type of NMSC [2, 3, 5]. It is characterized by slow progression, a low rate of metastasis, and long overall survival [5]. Eighty percent of lesions are located in the facial region [5]. If untreated, it can cause massive destruction of the surrounding tissues, and because it is so common, it remains a challenge to contemporary oncology. Dermoscopy (skin surface microscopy) is a non-invasive tool used for diagnosis of skin and mucous lesions. It provides a bridge between clinical and pathological examination. As a standardized diagnostic method, it is widely used for initial diagnosis, differentiation of various BCC subtypes, postoperative margin assessment, and early relapse detection [6].
High dose rate (HDR) brachytherapy is a treatment method that uses high-energy radioactive sources placed in proximity to the tumor. Thanks to computed planning, a precisely prescribed radiation dose can be delivered to the treated area with submillimeter precision. Contact surface brachytherapy is an organ-sparing method that has become more popular in recent years, especially in the treatment of skin cancers of the facial region in places where surgery would cause large disfigurements or loss of an organ [7, 8].
The current standard for monitoring the effects of BCC treatment is clinical examination and, in advanced cases, imaging studies including magnetic resonance or computed tomography. In some cases, assessment is made with ultrasonography or by using reflectance confocal microscopy. Histopathological examination is not routinely performed in the follow-up after treatment, so dermoscopy seems to have established itself as the most routinely used monitoring tool for now and the immediate future [9].
Changes in clinical and dermoscopic patterns during irradiation were analyzed and described by our investigation group in 2021 [9] in a prospective study. The current literature lacks more detailed descriptions of evolution in these patterns after brachytherapy.
Methods
A prospective, non-randomized study was designed. Thirty-nine patients diagnosed with high-risk basal cell carcinomas of the head and neck region qualified for HDR brachytherapy [Table 1]. Clinical and dermoscopic observations were performed to asses changes in patterns of the tumor and surrounding area after irradiation. The methodology of the clinical and dermoscopic analysis was based on our first short-term follow-up that investigated the evolution of clinical and dermoscopic patterns before every treatment fraction up to treatment end in the 3rd week [9]. The study protocol was approved by the Ethics Committee of the National Research Institute of Oncology, Gliwice Branch (reference number KB/430–41/20). The study was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided written informed consent to participate in the study, as well as for publication.
Patients’ Characteristics
Thirty-nine patients suffering from T1 and T2 [tumors below 4 cm in diameter, according to American Joint Committee of Cancer (AJCC)] [10] high-risk BCCs [according to the National Comprehensive Cancer Network (NCCN) 8th edition, 2017] [11], were disqualified from surgery because of age and coexisting comorbidities (11/39), poor expected cosmetic outcome (20/39), or due to patients’ preferences (8/39). Details of patients’ characteristics are presented in Table 1.
Inclusion Criteria
Inclusion criteria were: histopathologically confirmed basal cell carcinoma, tumor location in head and neck region (predominantly within H-facial zone), maximum tumor size 4 cm, disqualification from surgical excision, no prior irradiation in the tumor area, patient’s written informed consent, and age above 18 years.
Histopathological Assessment
All patients had histopathologically confirmed basal cell carcinomas using a standard stain with hematoxylin and eosin. Examination was performed by a qualified pathologist (M.S.). The predominant pattern in the microscopic image was reported according to the World Health Organization (WHO) classification current for 2018 [12].
Treatment
Patients were qualified for HDR brachytherapy using 192Ir isotope. Treatment was performed according to the protocol used by the Brachytherapy Department of Maria Sklodowska-Curie National Research Institute of Oncology (MSCNRIO) in Gliwice Branch [9]. The protocol was designed based on 30 years of experience in the field of skin brachytherapy, and has similarities with the Groupe Européen de Curiethérapie of the European Society for Radiotherapy & Oncology (GEC-ESTRO) recommendations for treating NMSCs [7]. All patients were treated with custom-made polyacrylamide mold applicators, individually built to firmly cover the treatment area. On the surface of the molds, catheters were placed 1 cm apart. Computed tomography was obtained with the applicator placed in the treatment area, and images were sent for treatment planning. Treatment was planned using Oncentra Masterplan software (Elekta, AB, version 4.6), and a dose of 45 Gy was prescribed for the tumor in 9 fractions of 5 Gy. Treatment was performed 3 times a week (Monday, Wednesday, Friday) on 3 consecutive weeks.
Observation
Clinical (macroscopic) and dermoscopic images were obtained before treatment (t1), on the day of the last fraction (t2), and at 24 weeks after the end of the treatment (t3). A DermLiteCam (3Gen, LLC, San Juan Capistrano, CA, USA) dermoscopic camera was used with tenfold magnification and polarized light, and every image was catalogued for further assessment. On every clinical picture, the presence (1) or absence (0) of 12 clinical features, typical for basal cell carcinomas were observed based on Tognetti and Lallas et al.’s classification (in authors’ modification) [6, 9, 13] were observed according to previously used and described methodology [9] (presented in Table 2).
Sixty one dermoscopic features typical for basal cell carcinoma were described according to the third consensus of the International Dermoscopic Society [14] (Table 3). Thirty-one non-neoplastic dermoscopic features of the area surrounding the tumor were also described according to expert consensus of the International Dermoscopic Society (IDS) [14]. Dermoscopic observations were performed by two independent dermoscopists (T.K. and G. K.-W.). In total, 603 photographs were obtained and a database of a total of 12,088 observations was analyzed. All patients suffered from acute radiation dermatitis, which was observed clinically on the area adjacent to the tumor and stratified according to Radiation Therapy Oncology Group (RTOG) criteria [16].
Statistical Analysis
Analysis of the 12,088 observations was performed. To evaluate the impact of the analyzed risk factors on binary skin diagnostic outcomes (dermoscopic features) in the observation periods, univariate logistic regression with random effects was applied. The statistical outcomes were expressed by a classic odds ratio (OR) together with a 95% confidence interval (95% CI) and a p-value. The computation was performed using the R statistical platform [17]. Tested p-values were adjusted using the Bonferroni correction.
Results
All patients suffered from high-risk basal cell carcinomas (39/39), 32 of them with primary diagnosed tumors (32/39), 7 were post-surgical relapses (7/39). Thirty-three were T1 tumors (33/39), and the rest were T2 (6/39) (according to AJCC) [10].
Clinical Follow-up
The clinical pre-treatment picture of observed BCCs (t1) was dominated by erosion or ulceration present among 30 of 39 patients, followed by crust or scale (29/39), short vessels (20/39), scar-like plaque (19/39), and telangiectasias (17/39). Macules, papules, or nodules were present in 15 cases (15/39).
Clinical evolution of the tumor at the end of treatment (t2) showed increased erosion and ulceration in the treated area (present in 36 of 39 patients), a decreased number of cases with short vessels and telangiectasias in comparison to pre-treatment evaluation (26 patients vs 37 patients), and a decreased number of macules, papules, and nodules (3 patients after vs 15 before treatment). Crust or scale was observed among 19 patients vs 29 before treatment.
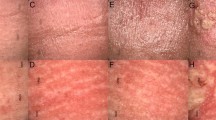
At 24 weeks (t3) after brachytherapy, only one erosion in the treated area was observed (patient reported trauma 1 day before examination, with no erosion prior to event). Telangiectasia or short vessels were observed among 15 patients. The number of macules, papules, or nodules decreased to 3. A small amount of crust or scale was observed in 9 cases. Examples of clinical changes are presented in Fig. 1.
Clinical observation of three basal cell carcinomas prior to treatment (A, D, G), at the end of brachytherapy (B, E, H), and 24 weeks after brachytherapy (C, F, I). Figures represent different types of BCC: postsurgical relapse (A), eroded nodular BCC of the nose (D) and nodular type (G). After treatment, at the peak of the early radiation toxicity ulceration, the treated area shows destruction of the tumor and grade 2 (G2) radiation dermatitis of surrounding healthy skin (B, E, H). After 6 months, no clinical patterns characteristic for BCC are visible, with some crust remaining in image I
Dermoscopic Follow-up
Dermoscopic pre-treatment images (t1) were dominated by linear, polymorphous, branched, and serpentine vessels (present among 30 of 39 cases). White or blue structureless zones were also observed (33/39), polychromatic structureless zones were observed in 12 cases. Erosion or ulceration was common (33/39). Non-neoplastic dermoscopic features (according to IDS) [15] were exhibited by linear (15/39) and branched (12/39) vessels, and yellow (17/39) and white (19/39) scale.
At the end of brachytherapy (t2) the number of linear, polymorphous, branched, and serpentine vessels decreased (17/39), as well as white or blue structureless zones (24/39). Polychromatic structureless zones were observed among 6 patients. Evolution of non-neoplastic features was exhibited by a reduction of linear (8/39) and branched (8/39) vessels. The amount of white (7/39) and yellow (13/39) scale was reduced.
At 24 weeks after treatment (t3), a white structureless area dominated the dermoscopic image (present among 32 patients of 39), followed by polychromatic structureless zones (8/39). Thin monomorphous vessels dominated (29/39), with polymorphous, branched vessels present among 2 patients. No ulceration or erosion was observed. The evolution of dermoscopic BCC features is presented in Fig. 2.
Dermoscopic images of basal cell carcinomas prior to treatment (A, D, G) at the end of brachytherapy (B, E, H), and 24 weeks after brachytherapy (C, F, I). Image A: post-surgical relapse is dominated by ulceration; image D: nodular type BCC with gray dots, linear, branched, and serpentine polymorphous vessels, and white and pink structureless zones. G: nodular type BCC with white, polychromatic structureless zones, and polymorphic linear vessels. BCC related features disappear at the end of brachytherapy with formation of ulceration (B, E, H). Twenty-four weeks after brachytherapy, BCC related dermoscopic features are reduced, there is visible telangiectasia (C, F) as late radiation morbidity, and white and pink structureless zones in the treated area, possibly due to formation of a scar
The dermoscopic observations proved that brachytherapy decreases the number of clinical and dermoscopic structures, both cancer related, and those not typical for BCC, as presented in Fig. 2.
The pattern of non-neoplastic features shows that the number of vessels reduced at the end of treatment returns to a pre-treatment level, with linear (23/39) and branched (14/39) vessels mostly not specifically arranged (15/39). White scale returned to a pre-treatment level (15/39), and the amount of yellow scale was reduced (3/39). Other features that emerged in the image were structureless areas (20/39), mostly white (17/39).
After 24 weeks, all patients achieved total clinical remission. The number of BCC-related dermoscopic patterns was reduced (Fig. 2C, F, I).
Acute radiation dermatitis was observed among all patients in the following grades (G): G1 (30/39), G2 (4/39), G3 (4/39), G4 (1/39), according to RTOG skin toxicity criteria [16]. Commonly, acute G1 toxicity presented as erythema of dry desquamation in the area surrounding the tumor. G4 toxicity was presented by ulceration of the perinasal area, which healed within 6 weeks. Late skin radiation toxicity after 6 months from treatment was stratified using the RTOG scale: G0 (8/39), G1 (25/39), G2 (6/39) (Fig. 1C, F, I). Depigmentation was the dominant symptom, with hair loss in the irradiated area and moderate telangiectasia as the dominant G2 presentation. No G3 late radiation toxicity was observed. The cosmetic effect was described as good or very good by all patients.
Typical clinical and dermoscopic presentation of the treated area 24 weeks after brachytherapy is presented in Fig. 3.
The list of statistically significant ORs (p < 0.05) of the influence of the analyzed risk factors on the skin diagnostic outcomes (univariate logistic regression with random effects) is reported in Table 4 and shown graphically in Fig. 4. Larger tumors have an increasing probability of dermoscopic presentation of telangiectasias (strongest correlation), polychromatic structureless zones, and clinically visible short vessels. The statistical interpretation of the ORs listed in Table 4 is as follows: the class of tumor size has a strong impact on the dermoscopic features reported; increasing the size of the tumor increases the risk of these changes by several times (from 3 for a branched vessel arrangement to almost 16 for telangiectasia).
A forest plot of statistically significant ORs (p < 0.05). Increasing tumor size correlates with higher possibility of observation of features presented above. The strongest correlation was observed towards telangiectasia and polychromatic structureless zones (univariate logistic regression with random effects)
Changes in the risk of the analyzed dermoscopic features in the subsequent stages of observation are reported in Table 5 and Fig. 5. Tested p-values were adjusted using the Bonferroni correction. Similarly, it can be seen that the risk of observation of clinical and dermoscopic features decreases with the next observation interval (t1–t3) by completing the estimated odds ratios (Table 2).
Discussion
The nodular subtype of BCC dominates in the general population (60–80%), followed by the superficial type (10–30%) [18]. Our study reflects that trend with 16 nodular type (16/39) and 10 superficial type (10/39) primary diagnosed tumors. The WHO classifies nodular and superficial types as tumors with low risk of recurrence [12]. Other BCC types present in our study—micronodular, infiltrating, and scleroderma-like, are considered high recurrence rate tumors. The NCCN stratifies basal cell carcinomas according to pathological subtype, tumor size, and location [11]. Head and neck BCCs are always considered to be high-risk tumors [11]. Curettage, electrodesiccation, imiquimod, and photodynamic therapy are not recommended treatment options for morphoeic, pigmented, or micronodular types, or for areas with higher risk of tumor survival and deep penetration (facial H-zone) [19].
Among the literature searched, only one published paper on dermoscopic follow-up in brachytherapy of BCCs was found [20]. Our group published results describing clinical and dermoscopic changes in basal cell carcinomas during HDR brachytherapy [9]. This study proved that every fraction of HDR brachytherapy reduces the number of clinical and dermoscopic patterns typical to BCC. The correlation was stronger for cancer-specific dermoscopic features than for non-neoplastic features [9]. In the treated area, erosion and ulceration was formed in the tumor bed [9]. Acute radiation dermatitis may cloud remaining dermoscopic features. From a radiobiological perspective, 24 weeks post-treatment time is considered the limit for acute radiation toxicity. All toxicity observed after that time is considered to be late toxicity [21]. In our study, at the 24-week time point, the acute radiation dermatitis had healed, and late radiation skin toxicity was described. All patients achieved clinical complete tumor remission, and all patients described the aesthetic effect as good or very good. Good cosmetic outcomes from brachytherapy were also reported in a previously published paper [8].
Typical dermoscopic evolution of irradiated basal cell carcinoma presents as progressing edema from pathological tumor vessels, with their subsequent erosion and destruction. At first, the amount of crust increases, but then diminishes, the number of structureless areas is reduced, and erythema dominates the vicinity [9]. In a previous pioneering study conducted by Navarrete-Dechent et al., [20] patients treated with brachytherapy were followed by dermoscopy, reflectance confocal microscopy, and ultrasound imaging. Dermoscopic follow-up showed diminishing numbers of arborizing vessels and a gradual increase of white color [20]. Our recent findings are consistent with this report where irradiated areas were dominated by large white structureless zones with short monomorphous vessels (Figs. 1 and 3). Dermoscopy was also proven to be a highly sensitive tool for detecting relapse or residual BCC. In the literature searched, dermoscopic monitoring of treated BCC was reported in topical non-ablative therapies. Apalla and colleagues [22] observed patients with BCCs treated topically with imiquimod or methylaminolaevulinate photodynamic therapy (PDT) and checked them 3 and 12 months after therapy. All patients with positive dermoscopic BCC features were biopsy positive. Furthermore, despite the different treatment, dermoscopic images of a cancer-free area resembles the post brachytherapy tumor bed, with the presence of large whitish areas representing upper dermal fibrosis and short, fine telangiectases; the superficial vascular plexus is more visible with post-treatment atrophy. Apalla et al. [22] also described the persistence of ovoid globular structures as markers of BCC early relapse. Aguilar and colleagues [23] indicated dermoscopic failure patterns among patients treated with imiquimod as another example of non-invasive treatment. The presence of arborizing telangiectasia, blue-gray ovoid nests, ulceration, perpendicular white lines (chrysalis) and white-red structureless areas correlated with poor response of the treated tumor. In the dermoscopic observation described by Husein El-Ahmed et al. [24] ulceration and neovascularization were the first dermoscopic features cleared, followed by blue-grey globules, leaf-like areas and large blue-grey ovoid nests, in the course of imiquimod topical therapy [24]. The authors correlated this with the depth of invasion and noticed that structures closer to the surface respond first [24].
Our present study showed no clinical or dermoscopic tumor persistence after 24 weeks after brachytherapy, nor any of the dermoscopic structures indicating potential residual disease presented above. Ionizing radiation as opposed to topical BCC therapies, including imiquimod or PDT, penetrates much deeper from the first dose to the depth planned by the radiologist [7]. Therefore, we observed much faster reduction of dermoscopic features, followed by ulceration in the tumor bed, than in previous studies [24].
Of interest will be the long-term clinical and dermoscopic observation at the 5-year evaluation in the context of potential recurrence or manifestation of residual disease features.
Other non-invasive diagnostic techniques are also valuable tools in diagnosis and post-treatment observation of BCCs. Optical coherence tomography (OCT) was proven to be a highly sensitive and specific diagnostic tool for BCC detection [25], while reflectance confocal microscopy (RCM) was described as superior to dermoscopy in treatment monitoring of BCC after radiotherapy [20]. Both methods were combined into a single diagnostic tool [26] that can further increase early detection of relapses. In this study, dermoscopic observations were not cross-referenced with OCT or RCM, which is a limitation of this study.
Conclusion
Dermoscopy is an efficient observational, easy-to-perform diagnostic method in the close-up monitoring and evaluation of BCC treated with HDR brachytherapy. Changes in clinical observations correlate with the dermoscopic evolution of radiation dermatitis and tumor destruction.
References
Global Cancer Observatory, report on year 2020 https://gco.iarc.fr/today/, Accessed July 14 2022.
Aggarwal P, Knabel P, Fleischer A. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85(2):388–95.
Wojciechowska U, Didkowska J, Michałek J et al. Cancer in Poland 2018, 16–21.
Zanetti R, Rosso S, Martinez C, Mossotti R, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Cancer. 2006;13(94):743–51.
Ciążyńska M, Kamińska-Winciorek G, Lange D, et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep. 2021;11:4337.
Lallas A, Apalla Z, Argenziano G, Longo C, Moscarella E, Specchio F, Zalaudek I. The dermatoscopic universe of basal cell carcinoma. Dermatol Practical Conceptual. 2014;4:11–24.
Guinot JL, Rembielak A, Perez-Calatayud J, Rodríguez-Villalba S, Skowronek J, Tagliaferri L, Guix B, Gonzalez-Perez V, Valentini V, Kovacs G; GEC ESTRO. GEC-ESTRO ACROP recommendations in skin brachytherapy. RadiotherOncol. 2018;126:377–385.
Krzysztofiak T, Kamińska-Winciorek G, Pilśniak A, Wojcieszek P High dose rate brachytherapy in non-melanoma skin cancer—Systematic review. Dermatologic Therapy. 2022;35(9).
Krzysztofiak T, Kamińska-Winciorek G, Tukiendorf A, Suchorzepka M, Wojcieszek P. Basal cell carcinoma treated with high dose rate (HDR) brachytherapy—early evaluation of clinical and dermoscopic patterns during irradiation. Cancers. 2021;13:5188.
American Joint Committee on Cancer. Cutaneous Squamous Cell Carcinoma of the Head and Neck. In: AJCC Cancer Staging Manual. 8th ed. Springer, New York, US, 2017;171–181.
NCCN guidelines Non Melanoma Skin Cancer, 2022, V.2. Available online: https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations. Accessed on 17 July 2021.
Messina J, Epstein EH Jr, Kossard S, McKenzie C, Patel RM, Patterson JW, Scolyer RA. WHO classification of skin tumours, Basal cell carcinoma, 2018;26–34.
Tognetti L, Cinotti E, Fiorani D, et al. Long-term therapy of multiple basal cell carcinomas: Clinicodermoscopic score for monitoring of intermittent vismodegib treatment. Dermatol Ther. 2019;32: e13097.
Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093–106.
Errichetti E, Zalaudek I, Kittler H, et al Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol 2020;182;454–467.
Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–6.
R Core Team (2020). A language and environment for statistical computing. Version 4.2.1. Vienna, Austria
Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12–7.
Peris K, Fargnoli M, Garbe C, et al. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. Eur J Cancer. 2019;118:10–34.
Navarrete-Dechent C, Cordova M, Liopyris K, et al. In vivo imaging characterization of basal cell carcinoma and cutaneous response to high-dose ionizing radiation therapy: a prospective study of reflectance confocal microscopy, dermoscopy, and ultrasonography. J Am Acad Dermatol. 2021;84:1575–84.
Dörr W Radiobiology of tissue reactions. Annals of the ICRP. 2015, 44 (1_suppl), 58–68.
Apalla Z, Lallas A, Tzellos T, et al. Applicability of dermoscopy for evaluation of patients’ response to nonablative therapies for the treatment of superficial basal cell carcinoma. Br J Dermatol. 2014;170:809–15.
Aróstegui Aguilar J, Hervella Garcés M, Yanguas Bayona JI, Azcona Rodríguez M. Dermoscopic signs as predictors of non-response to imiquimod treatment in superficial basal cell carcinoma. An Sist Sanit Navar. 2019;42:303–7.
Husein-ElAhmed H, Fernandez-Pugnaire MA. Dermatoscopy-guided therapy of pigmented basal cell carcinoma with imiquimod. An Bras Dermatol. 2016;91:764–9.
Reddy N, Nguyen BT. The utility of optical coherence tomography for diagnosis of basal cell carcinoma: a quantitative review. Br J Dermatol. 2019;180:475–83.
Iftimia N, Sahu A, Cordova M, et al. The potential utility of integrated reflectance confocal microscopy-optical coherence tomography for guiding triage and therapy of basal cell carcinomas. J Cancer. 2020;18:6019–24.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study. Publication of this article and the Rapid Service Fee was funded by the National Research Institute of Oncology (MSCNRIO), Gliwice Branch.
Medical Writing and Editorial Assistance
The authors did not have medical writing or editorial assistance for this paper.
Author Contributions
Conceptualization, Krzysztofiak Tomasz and Kamińska-Winciorek Grażyna; Data curation, Krzysztofiak Tomasz, Kamińska-Winciorek Grażyna, Tukiendorf Andrzej, Suchorzepka Magdalena; Formal analysis, Krzysztofiak Tomasz, Kamińska-Winciorek Grażyna, Tukiendorf Andrzej, Suchorzepka Magdalena; Funding acquisition, Kamińska-Winciorek Grażyna; Investigation, Krzysztofiak Tomasz, Tukiendorf Andrzej, Suchorzepka Magdalena; Methodology, Krzysztofiak Tomasz, Kamińska-Winciorek Grażyna, Tukiendorf Andrzej; Project administration, Krzysztofiak Tomasz, Kamińska-Winciorek Grażyna, Wojcieszek Piotr; Resources, Krzysztofiak Tomasz, Tukiendorf Andrzej, Suchorzepka Magdalena; Software, Tukiendorf Andrzej; Supervision, Kamińska-Winciorek Grażyna, and Wojcieszek Piotr; Validation, Krzysztofiak Tomasz, Kamińska-Winciorek Grażyna, Tukiendorf Andrzej; Visualization, Krzysztofiak Tomasz, Kamińska-Winciorek Grażyna, and Suchorzepka Magdalena; Writing—original draft, Krzysztofiak Tomasz, Kamińska-Winciorek Grażyna, Tukiendorf Andrzej; Writing—review & editing, Krzysztofiak Tomasz, Kamińska-Winciorek Grażyna, Tukiendorf Andrzej, Suchorzepka Magdalena, and Wojcieszek Piotr.
Disclosures
The authors have no conflicts of interest to declare.
Compliance with Ethics Guidelines
The study protocol was approved by the Ethics Committee of the National Research Institute of Oncology, Gliwice Branch (reference number KB/430–41/20). The study was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments.
Data Availability
The data that support the findings of this study are not publicly available due to containing information that could compromise the privacy of research participants, but are available from the corresponding author [Tomasz Krzysztofiak].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Krzysztofiak, T., Suchorzepka, M., Tukiendorf, A. et al. Basal Cell Carcinoma After High Dose Rate Brachytherapy: Medium-term Dermoscopic Evaluation of Cancer’s Response. Dermatol Ther (Heidelb) 13, 2063–2078 (2023). https://doi.org/10.1007/s13555-023-00981-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00981-5