Abstract
Background and Objectives
The novel tyrosine kinase inhibitor (TKI) dasatinib, a multitarget inhibitor of Bcr-Abl and Src family kinases, has been licensed for the treatment of Ph+ acute lymphoblastic leukemia and chronic myeloid leukemia. Many citrus-based foods include the flavonoid naringenin, which is commonly available. Dasatinib is a Cyp3a4, P-gp, and Bcrp1 substrate, which makes it sensitive to potential food–drug interactions. The concurrent use of naringenin may change the pharmacokinetics of dasatinib, which could result in adverse effects and toxicity. The present investigation examined the impact of naringenin on the pharmacokinetics interactions of DAS and proposes a possible interaction mechanism in Wistar rats.
Methods
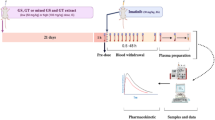
Rats were provided with a single oral dose of dasatinib (25 mg/kg) with or without naringenin pretreatment (150 mg/kg p.o. daily for 7 days, n = 6 in each group). Dasatinib was quantified in plasma by UHPLC MS/MS assay. Noncompartmental analysis was used to compute the pharmacokinetic parameters, and immunoblot was used to assess the protein expression in the hepatic and intestinal tissues.
Results
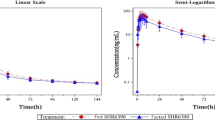
Following 7 days of naringenin pretreatment, the plasma mean concentration of dasatinib was enhanced compared with without pretreatment. In rats that were pretreated with naringenin, the pharmacokinetics of the orally administered dasatinib (25 mg/kg) was shown to be significantly different from that of dasatinib given without pretreatment (p < 0.05). There was a significant enhancement in pharmacokinetic parameters elimination half-life (T1/2), time to maximum concentration ( Tmax), maximum concentration )Cmax), area under the concentration–time curve (AUC0–t), area under the moment curve (AUMC0–∞), and mean residence time (MRT) by 28.41%, 50%, 103.54%, 72.64%, 115.08%, and 15.19%, respectively (p < 0.05) and suppression in elimination rate constant (Kel), volume of distribution (Vd), and clearance (CL) by 21.09%, 31.13%, and 46.25%, respectively, in comparison with dasatinib alone group (p < 0.05). The enhancement in dasatinib bioavailability and systemic exposure resulted from the significant inhibition of Cyp3a2, Mdr1/P-gp, and Bcrp1 expression and suppression of the dasatinib hepatic and intestinal metabolism, which enhanced the rate of dasatinib absorption and decreased its elimination.
Conclusion
Concurrent use of naringenin-containing supplements, herbs, or foods with dasatinib may cause serious and potentially life-threatening drug interactions. Further studies are necessary to determine the clinical significance of these findings.


Similar content being viewed by others
References
Paul MK, Mukhopadhyay AK. Tyrosine kinase - role and significance in cancer. Int J Med Sci. 2004;1:101–15.
Teo YL, Ho HK, Chan A. Metabolism-related pharmacokinetic drug-drug interactions with tyrosine kinase inhibitors: current understanding, challenges and recommendations. Br J Clin Pharmacol. 2015;79:241–53.
Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10:470–81.
Bonvin A, Mesnil A, Nicolini FE, Cotte L, Michallet M, Descotes J, et al. Dasatinib-induced acute hepatitis. Leuk Lymphoma. 2008;49:1630–2.
Rochat B, Fayet A, Widmer N, Lahrichi SL, Pesse B, Decosterd LA, et al. Imatinib metabolite profiling in parallel to imatinib quantification in plasma of treated patients using liquid chromatography-mass spectrometry. J Mass Spectrom. 2008;43:736–52.
Kamath AV, Wang J, Lee FY, Marathe PH. Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): a potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemother Pharmacol. 2008;61:365–76.
Keam S. Dasatinib. Bio-Drugs. 2008;22:59–69.
Squibb BM. Sprycel (dasatinib). https://news.bms.com/news/details/2017/US-Food-and-Drug-Administration-Expands-Approval-of-Sprycel-dasatinib-to-Include-Treatment-of-Children-with-Philadelphia-Chromosome-Positive-Chronic-Myeloid-Leukemia-in-Chronic-Phase/default.aspx. Accessed Oct 2023. 2017
Steegmann JL, Baccarani M, Breccia M, Casado LF, Garcia-Gutierrez V, Hochhaus A, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–71.
Herviou P, Thivat E, Richard D, Roche L, Dohou J, Pouget M, et al. Therapeutic drug monitoring and tyrosine kinase inhibitors. Oncol Lett. 2016;12:1223–32.
Wang B, Shen J, Zhou Q, Meng D, He Y, Chen F, et al. Effects of naringenin on the pharmacokinetics of tofacitinib in rats. Pharm Biol. 2020;58:225–30.
Gupta P, Chow V, Wang R, Kaplan I, Chan G, Alvey C, et al. Evaluation of the effect of fluconazole and ketoconazole on the pharmacokinetics of tofacitinib in healthy adult subjects. Clin Pharmacol Drug Dev. 2014;3:72–7.
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–46.
Zeng W, Jin L, Zhang F, Zhang C, Liang W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol Res. 2018;135:122–6.
Yang Y, Qi J, Zhang M, Chen P, Liu Y, Sun X, et al. The cardioprotective effects and mechanisms of naringenin in myocardial ischemia based on network pharmacology and experiment verification. Front Pharmacol. 2022;13: 954555.
Salehi B, Fokou PVT, Sharifi-Rad M, Zucca P, Pezzani R, Martins N, et al. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals (Basel). 2019. https://doi.org/10.3390/ph12010011.
Alzoman NZ, Maher HM, Shehata SM, Abanmy NO. UPLC-MS/MS study of the effect of dandelion root extract on the plasma levels of the selected irreversible tyrosine kinase inhibitors dasatinib, imatinib and nilotinib in rats: potential risk of pharmacokinetic interactions. Biomed Chromatogr. 2019;33: e4674.
Zhou Y, Wang S, Ding T, Chen M, Wang L, Wu M, et al. Evaluation of the effect of apatinib (YN968D1) on cytochrome P450 enzymes with cocktail probe drugs in rats by UPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;973C:68–75.
Liu L, Li P, Qiao L, Li X. Effects of astragaloside IV on the pharmacokinetics of puerarin in rats. Xenobiotica. 2019;49:1173–7.
Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice-drug interactions. Br J Clin Pharmacol. 1998;46:101–10.
Lundahl J, Regardh CG, Edgar B, Johnsson G. Effects of grapefruit juice ingestion–pharmacokinetics and haemodynamics of intravenously and orally administered felodipine in healthy men. Eur J Clin Pharmacol. 1997;52:139–45.
Pingili RB, Vemulapalli S, Dirisala VR, Mullapudi SS, Gullapalli Y, Kilaru NB. Effect of naringenin on the pharmacokinetics of metoprolol succinate in rats. Xenobiotica. 2021;51:926–32.
Pingili R, Vemulapalli S, Mullapudi SS, Nuthakki S, Pendyala S, Kilaru N. Pharmacokinetic interaction study between flavanones (hesperetin, naringenin) and rasagiline mesylate in wistar rats. Drug Dev Ind Pharm. 2016;42:1110–7.
Rosenberg EI, Genao I, Chen I, Mechaber AJ, Wood JA, Faselis CJ, et al. Complementary and alternative medicine use by primary care patients with chronic pain. Pain Med. 2008;9:1065–72.
Hassen G, Belete G, Carrera KG, Iriowen RO, Araya H, Alemu T, et al. Clinical implications of herbal supplements in conventional medical practice: a US perspective. Cureus. 2022;14: e26893.
Raish M, Ahmad A, Shahid M, Jardan YAB, Ahad A, Kalam MA, et al. Effects of apigenin on pharmacokinetics of dasatinib and probable interaction mechanism. Molecules. 2023;28:1602.
Meesters R, Voswinkel SJJAB. Bioanalytical method development and validation: from the USFDA 2001 to the USFDA 2018 guidance for industry. J Appl Bioanal. 2018;4:67–73.
USFDA. Guidance for industry, bioanalytical method validation, US department of health and human services, food and drug administration, center for drug evaluation and research (CDER), Center for veterinary medicine (CMV), May 2018. Document available at https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf. Accessed Sep 2023. 2018
Ezzeldin E, Iqbal M, Herqash RN, ElNahhas T. Simultaneous quantitative determination of seven novel tyrosine kinase inhibitors in plasma by a validated UPLC-MS/MS method and its application to human microsomal metabolic stability study. J Chromatogr B Analyt Technol Biomed Life Sci. 2020;1136: 121851.
Korashy HM, Ansari MA, Maayah ZH, Imam F, Raish M, Attafi IM, et al. Differential effects of sunitinib on the expression profiles of xenobiotic-metabolizing enzymes and transporters in rat liver and kidneys. Basic Clin Pharmacol Toxicol. 2016;119:173–83.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8.
Fujita KI, Ishida H, Kubota Y, Sasaki Y. Toxicities of receptor tyrosine kinase inhibitors in cancer pharmacotherapy: management with clinical pharmacology. Curr Drug Metab. 2017;18:186–98.
Zhao Q, Wu ZE, Li B, Li F. Recent advances in metabolism and toxicity of tyrosine kinase inhibitors. Pharmacol Ther. 2022;237: 108256.
Fasinu P, Choonara YE, Khan RA, Du Toit LC, Kumar P, Ndesendo VM, et al. Flavonoids and polymer derivatives as CYP3A4 inhibitors for improved oral drug bioavailability. J Pharm Sci. 2013;102:541–55.
Fasinu PS, Rapp GK. Herbal interaction with chemotherapeutic drugs-a focus on clinically significant findings. Front Oncol. 2019;9:1356.
Rashrash M, Schommer JC, Brown LM. Prevalence and predictors of herbal medicine use among adults in the United States. J Patient Exp. 2017;4:108–13.
Hill J, Seguin R, Manda A, Chikasema M, Vaz O, Li Q, et al. Prevalence of traditional, complementary, and alternative medicine (TCAM) among adult cancer patients in Malawi. Cancer Causes Control. 2022;33:1047–57.
Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther. 2009;330:956–63.
van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35:692–706.
Haouala A, Widmer N, Duchosal MA, Montemurro M, Buclin T, Decosterd LA. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood. 2011;117:e75-87.
Gardiner P, Phillips R, Shaughnessy AF. Herbal and dietary supplement–drug interactions in patients with chronic illnesses. Am Fam Physician. 2008;77:73–8.
Miller MF, Bellizzi KM, Sufian M, Ambs AH, Goldstein MS, Ballard-Barbash R. Dietary supplement use in individuals living with cancer and other chronic conditions: a population-based study. J Am Diet Assoc. 2008;108:483–94.
Fleisher B, Unum J, Shao J, An G. Ingredients in fruit juices interact with dasatinib through inhibition of BCRP: a new mechanism of beverage-drug interaction. J Pharm Sci. 2015;104:266–75.
D’Cunha R, Bae S, Murry DJ, An G. TKI combination therapy: strategy to enhance dasatinib uptake by inhibiting Pgp- and BCRP-mediated efflux. Biopharm Drug Dispos. 2016;37:397–408.
Zakharyants AA, Burmistrova OA, Poloznikov AA. The use of human liver cell model and cytochrome P450 substrate-inhibitor panel for studies of dasatinib and warfarin interactions. Bull Exp Biol Med. 2017;162:515–9.
Abdelgalil AA, Alam MA, Raish M, Mohammed IE, Hassan Mohammed AE, Ansari MA, et al. Dasatinib significantly reduced in vivo exposure to cyclosporine in a rat model: the possible involvement of CYP3A induction. Pharmacol Rep. 2019;71:201–5.
Luo X, Xue X, Li T, Zhang Y, Huang L, Cheng G. Differential impacts of azole antifungal drugs on the pharmacokinetic profiles of dasatinib in rats by LC-MS-MS. Curr Drug Metab. 2020;21:1022–30.
Ho PC, Saville DJ, Coville PF, Wanwimolruk S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharm Acta Helv. 2000;74:379–85.
Takahata T, Ookawa K, Suto K, Tanaka M, Yano H, Nakashima O, et al. Chemosensitivity determinants of irinotecan hydrochloride in hepatocellular carcinoma cell lines. Basic Clin Pharmacol Toxicol. 2008;102:399–407.
Surya Sandeep M, Sridhar V, Puneeth Y, Ravindra Babu P, Naveen BK. Enhanced oral bioavailability of felodipine by naringenin in Wistar rats and inhibition of P-glycoprotein in everted rat gut sacs in vitro. Drug Dev Ind Pharm. 2014;40:1371–7.
Kaci H, Bodnarova S, Fliszar-Nyul E, Lemli B, Pelantova H, Valentova K, et al. Interaction of luteolin, naringenin, and their sulfate and glucuronide conjugates with human serum albumin, cytochrome P450 (CYP2C9, CYP2C19, and CYP3A4) enzymes and organic anion transporting polypeptide (OATP1B1 and OATP2B1) transporters. Biomed Pharmacother. 2023;157: 114078.
Christopher LJ, Cui D, Wu C, Luo R, Manning JA, Bonacorsi SJ, et al. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab Dispos. 2008;36:1357–64.
Ortiz-Andrade RR, Sanchez-Salgado JC, Navarrete-Vazquez G, Webster SP, Binnie M, Garcia-Jimenez S, et al. Antidiabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra-pancreatic glucose regulation. Diabetes Obes Metab. 2008;10:1097–104.
Rani N, Bharti S, Krishnamurthy B, Bhatia J, Sharma C, Kamal MA, et al. Pharmacological properties and therapeutic potential of naringenin: a citrus flavonoid of pharmaceutical promise. Curr Pharm Des. 2016;22:4341–59.
Galluzzo P, Ascenzi P, Bulzomi P, Marino M. The nutritional flavanone naringenin triggers antiestrogenic effects by regulating estrogen receptor alpha-palmitoylation. Endocrinology. 2008;149:2567–75.
Shahid M, Ahmad A, Raish M, Bin Jardan YA, Alkharfy KM, Ahad A, et al. Herb-drug interaction: effect of sinapic acid on the pharmacokinetics of dasatinib in rats. Saudi Pharm J. 2023;31: 101819.
Edwards DJ, Bernier SM. Naringin and naringenin are not the primary CYP3A inhibitors in grapefruit juice. Life Sci. 1996;59:1025–30.
Eagling VA, Profit L, Back DJ. Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the HIV-1 protease inhibitor saquinavir by grapefruit juice components. Br J Clin Pharmacol. 1999;48:543–52.
Zhang S, Yang X, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004;21:1263–73.
Ali MM, Agha FG, El-Sammad NM, Hassan SK. Modulation of anticancer drug-induced P-glycoprotein expression by naringin. Z Naturforsch C J Biosci. 2009;64:109–16.
Wang L, Christopher LJ, Cui D, Li W, Iyer R, Humphreys WG, et al. Identification of the human enzymes involved in the oxidative metabolism of dasatinib: an effective approach for determining metabolite formation kinetics. Drug Metab Dispos. 2008;36:1828–39.
Fan X, Bai J, Zhao S, Hu M, Sun Y, Wang B, et al. Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): from library screening to biological evaluation to structure-activity relationship. Toxicol In Vitro. 2019;61: 104642.
Burkina V, Zlabek V, Halsne R, Ropstad E, Zamaratskaia G. In vitro effects of the citrus flavonoids diosmin, naringenin and naringin on the hepatic drug-metabolizing CYP3A enzyme in human, pig, mouse and fish. Biochem Pharmacol. 2016;110–111:109–16.
Diaconu CH, Cuciureanu M, Vlase L, Cuciureanu R. Food-drug interactions: grapefruit juice. Rev Med Chir Soc Med Nat Iasi. 2011;115:245–50.
Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64:4346–52.
de Castro WV, Mertens-Talcott S, Derendorf H, Butterweck V. Grapefruit juice-drug interactions: grapefruit juice and its components inhibit P-glycoprotein (ABCB1) mediated transport of talinolol in Caco-2 cells. J Pharm Sci. 2007;96:2808–17.
Acknowledgements
The authors are grateful to the Researchers Supporting Project Number (RSPD2024R541) at King Saud University, Riyadh, Saudi Arabia for funding this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional Review Board Statement
The research was approved by the Research Ethics Committee of King Saud University College of Pharmacy Riyadh, Saudi Arabia (KSU-SE-21-58).
Informed Consent
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Data Availability
The data generated from the study have been presented in the manuscript.
Author Contributions
MR and AA: conceptualization, formal analysis, and writing and reviewing original draft. BAK: experimental work. YABJ: methodology and supervision. AA, MI: formal analysis. KMA: writing and reviewing original draft. FIA-J: conceptualization, supervision, funding acquisition, writing–review and editing.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raish, M., Ahmad, A., Karim, B.A. et al. Pharmacokinetics of Dasatinib in Rats: a Potential Food–Drug Interaction with Naringenin. Eur J Drug Metab Pharmacokinet 49, 239–247 (2024). https://doi.org/10.1007/s13318-024-00881-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-024-00881-9