Abstract
Background and Objective
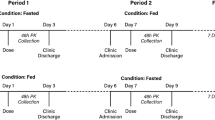
CC-292 is a potent, selective, orally administered small molecule inhibitor of Bruton’s tyrosine kinase (BTK). To support the clinical investigation of CC-292, a randomized, seven-treatment, seven-period, crossover study was conducted to assess the relative bioavailability, pH effect, food effect, and dose-proportionality of two formulated tablets of CC-292.
Methods
Healthy subjects (n = 24) were enrolled in the study and randomly assigned into different treatment sequences. Blood samples were collected at pre-specified time points to measure the drug concentrations in plasma. Statistical analyses were performed to compare the pharmacokinetics of CC-292 under different conditions.
Results
The relative bioavailability of the newly developed formulation [spray-dried dispersion (SDD)] to the reference formulation (P22) was 1.24. When a single dose of CC-292 SDD tablet was administered under fed conditions, the area under the plasma concentration–time curve from time zero to infinity (AUC∞) increased by 10.9% and the maximum plasma drug concentration Cmax) decreased by 19.4% compared to when CC-292 was administered under fasted conditions. When a single dose of CC-292 SDD tablet was administered after multiple doses of omeprazole, the area under the plasma concentration–time curve from time zero to infinity (AUC∞) decreased by 36.8% and the maximum plasma drug concentration Cmax) decreased by 48.1% compared to when CC-292 was administered alone. Over a dose range of 100–300 mg (SDD formulation), CC-292 exhibited more than dose-proportional increases of drug exposures.
Conclusions
CC-292 was well tolerated when administered to healthy subjects as single oral doses under all conditions. Food intake had no clinically relevant impact on CC-292 pharmacokinetics compared to fasted conditions. Therefore, CC-292 can be administered with or without food. Co-administration of CC-292 with multiple doses of omeprazole (40 mg) decreased the pharmacokinetic exposure of CC-292. However, the effect was not clinically relevant.
Clinical Trials Registration
NCT02433457





Similar content being viewed by others
References
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88. https://doi.org/10.1056/NEJMoa0706383.
Gururajan M, Jennings CD, Bondada S. Cutting edge: constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J Immunol. 2006;176(10):5715–9. https://doi.org/10.4049/jimmunol.176.10.5715.
Edwards JC, Cambridge G. Prospects for B-cell-targeted therapy in autoimmune disease. Rheumatology (Oxford). 2005;44(2):151–6. https://doi.org/10.1093/rheumatology/keh446.
Silverman GJ, Weisman S. Rituximab therapy and autoimmune disorders: prospects for anti-B cell therapy. Arthitis Rheum. 2003;48(6):1484–92. https://doi.org/10.1002/art.10947.
Küppers R. Somatic hypermutation and B cell receptor selection in normal and transformed human B cells. Ann NY Acad Sci. 2003;987:173–9. https://doi.org/10.1111/j.1749-6632.2003.tb06046.x.
Smith CI, Baskin B, Humire-Greiff P, Zhou JN, Olsson PG, Maniar HS, Kjellén P, Lambris JD, Chistensson B, Hammarström L, et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J Immunol. 1994;152(2):557–65.
Mohamed AJ, Yu L, Bäckesjö CM, Vargas L, Faryal R, Aints A, Chistensson B, Berglöf A, Vihinen M, Nore BF, Smith CI. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev. 2009;228(1):58–73. https://doi.org/10.1111/j.1600-065X.2008.00741.x.
Akinleye A, Chen Y, Mukhi N, Song Y, Liu D. Ibrutinib and novel BTK inhibitors in clinical development. J Hematol Oncol. 2013;6:59. https://doi.org/10.1186/1756-8722-6-59.
Evans EK, Tester R, Aslanian S, Karp R, Sheets M, Labenski MT, Witowski SR, Lounsbury H, Chaturvedi P, Mazdiyasni H, Zhu Z, Nacht M, Freed MI, Petter RC, Dubrovskiy A, Singh J, Westlin WF. Inhibition of Btk with CC-292 provides early pharmacodynamic assessment of activity in mice and humans. J Pharmacol Exp Ther. 2013;346(2):219–28. https://doi.org/10.1124/jpet.113.203489.
Schafer PH, Kivitz AJ, Ma J, Korish S, Sutherland D, Li L, Azaryan A, Kosek J, Adams M, Capone L, Hur EM, Hough DR, Ringheim GE. Spebrutinib (CC-292) affects markers of B cell activation, chemotaxis, and osteoclasts in patients with rheumatoid arthitis: results from a mechanistic study. Rheumatol Ther. 2020;7(1):101–19. https://doi.org/10.1007/s40744-019-00182-7.
Brown JR, Harb WA, Hill BT, Gabrilove J, Sharman JP, Scheeder MT, Barr PM, Foran JM, Miller TP, Burger JA, Kelly KR, Mahadevan D, Ma S, Li Y, Pierce DW, Barnett E, Marine J, Miranda M, Azaryan A, Yu X, Nava-Parada P, Mei J, Kipps TJ. Phase I study of single-agent CC-292, a highly selective Bruton’s tyrosine kinase inhibitor, in relapsed/refractory chonic lymphocytic leukemia. Haematologica. 2016;101(7):e295-298. https://doi.org/10.3324/haematol.2015.140806.
Li Y, Ramírez-Valle F, Xue Y, Ventura JI, Gouedard O, Mei J, Takeshita K, Palmisano M, Zhou S. Population pharmacokinetics and exposure response assessment of CC-292, a potent BTK inhibitor, in patients with chonic lymphocytic leukemia. J Clin Pharmacol. 2017;57(10):1279–89. https://doi.org/10.1002/jcph.923.
FDA (2002) Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. Available from https://www.fdagov/files/drugs/published/Guidance-for-Industry-Bioavailability-and-Bioequivalence-Studies-for-Orally-Administered-Drug-Products---General-ConsiderationsPDF Accessed Mar 18, 22
FDA (2014) Guidance for industry: bioavailability and bioequivalence studies submitted in NDAs or INDs—General Considerations Available from https://www.fdagov/media/88254/download. Accessed Mar 18, 22
Smelick GS, Heffron TP, Chu L, Dean B, West DA, Duvall SL, Lum BL, Budha N, Holden SN, Benet LZ, Frymoyer A, Dresser MJ, Ware JA. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm. 2013;10(11):4055–62. https://doi.org/10.1021/mp400403s.
Miner PB Jr, Allgood LD, Grender JM. Comparison of gastric pH with omeprazole magnesium 20.6 mg (Prilosec OTC) o.m. famotidine 10 mg (Pepcid AC) b.d. and famotidine 20 mg b.d. over 14 days of treatment. Aliment Pharmacol Ther. 2007;25(1):103–9. https://doi.org/10.1111/j.1365-2036.2006.03129.x.
Fang AF, Damle BD, LaBadie RR, Crownover PH, Hewlett D Jr, Glue PW. Significant decrease in nelfinavir systemic exposure after omeprazole coadministration in healthy subjects. Pharmacotherapy. 2008;28(1):42–50. https://doi.org/10.1592/phco.28.1.42.
Tappouni HL, Rublein JC, Donovan BJ, Hollowell SB, Tien HC, Min SS, Theodore D, Rezk NL, Smith PC, Tallman MN, Raasch RH, Kashuba AD. Effect of omeprazole on the plasma concentrations of indinavir when administered alone and in combination with ritonavir. Am J Health Syst Pharm. 2008;65(5):422–8. https://doi.org/10.2146/ajhp070226.
Zhu L, Persson A, Mahnke L, Eley T, Li T, Xu X, Agarwala S, Dragone J, Bertz R. Effect of low-dose omeprazole (20 mg daily) on the pharmacokinetics of multiple-dose atazanavir with ritonavir in healthy subjects. J Clin Pharmacol. 2011;51(3):368–77. https://doi.org/10.1177/0091270010367651.
Crauwels H, van Heeswijk RP, Stevens M, Buelens A, Vanveggel S, Boven K, Hoetelmans R. Clinical perspective on drug-drug interactions with the non-nucleoside reverse transcriptase inhibitor rilpivirine. AIDS Rev. 2013;15(2):87–101.
Jaruratanasirikul S, Sriwiriyajan S. Effect of omeprazole on the pharmacokinetics of itraconazole. Eur J Clin Pharmacol. 1998;54(2):159–61. https://doi.org/10.1007/s002280050438.
Dixit RK, Chawla AB, Kumar N, Garg SK. Effect of omeprazole on the pharmacokinetics of sustained-release carbamazepine in healthy male volunteers. Methods Find Exp Clin Pharmacol. 2001;23(1):37–9. https://doi.org/10.1358/mf.2001.23.1.619178.
Shirasaka Y, Sager JE, Lutz JD, Davis C, Isoherranen N. Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions. Drug Metab Dispos. 2013;41(7):1414–24. https://doi.org/10.1124/dmd.113.051722.
FDA (2019) Guidance for Industry: assessing the effects of food on drugs in INDs and NDAs—clinical pharmacology considerations. Available from https://www.fdagov/media/121313/download. Accessed Mar 18, 22
Klein CE, Chiu YL, Awni W, Zhu T, Heuser RS, Doan T, Breitenbach J, Morris JB, Brun SC, Hanna GJ. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J Acquir Immune Defic Syndr. 2007;44(4):401–10. https://doi.org/10.1097/QAI.0b013e31803133c5.
Klueglich M, Ring A, Scheuerer S, Trommeshauser D, Schuijt C, Liepold B, Berndl G. Ibuprofen extrudate, a novel, rapidly dissolving ibuprofen formulation: relative bioavailability compared to ibuprofen lysinate and regular ibuprofen, and food effect on all formulations. J Clin Pharmacol. 2005;45(9):1055–61. https://doi.org/10.1177/0091270005279579.
O’Shea JP, Holm R, O’Driscoll CM, Griffin BT. Food for thought: formulating away the food effect—a PEARRL review. J Pharm Pharmacol. 2019;71(4):510–35. https://doi.org/10.1111/jphp.12957.
Ludden TM. Nonlinear pharmacokinetics. Clin Pharmacokinet. 1991;20(6):429–46. https://doi.org/10.2165/00003088-199120060-00001.
Acknowledgements
The authors thank all study participants, their families and clinical study team members.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was sponsored and funded by Bristol Myers Squibb.
Competing Interests
Y.C., L.L., Y.X., S.Z. and Y.L. are employees and hold equity ownership in Bristol Myers Squibb.
Consent for Publication
Not applicable.
Ethics Approval
The study was conducted in accordance with the applicable United States (US) Code of Federal Regulations (CFR) governing the Protection of Human Subjects (21 CFR 50, 1980), Financial Disclosure by Clinical Investigators (21 CFR 54, 1998), Institutional Review Board (IRB) (21 CFR 56, 1981), the Investigational New Drug Application (21 CFR 312, 1987), and Applications for Food and Drug Administration Approval to Market a New Drug (21 CFR 314, 1985). As such, these sections of US Title 21 CFR, along with the applicable International Conference on Harmonisation Guidelines (ICH, E6, 1997), are commonly known as Good Clinical Practice (GCP), which are consistent with the Declaration of Helsinki (World Medical Association, 2008). The study was approved by the Schulman Associates Independent Review Board (Cincinnati, Ohio, USA).
Consent to Participate
The investigator obtained written informed consent from the subjects prior to any study-related procedures.
Code Availability
Not applicable.
Availability of Data and Material
The datasets are available from the corresponding author upon request.
Authors' Contributions
Y.L. and L.L. contributed to conception and design; Y.L. and Y.X. contributed to acquisition of data; Y.C., L.L., S.Z. and Y.L. contributed to analysis; Y.C., S.Z. and Y.L. drafted and revised the article. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, Y., Liu, L., Xue, Y. et al. An Open Label, Phase 1, Randomized, Seven-treatment, Seven-period, Crossover Study to Assess the Relative Bioavailability, pH Effect, Food Effect, and Dose Proportionality of CC-292, a Potent and Orally Available Bruton’s Tyrosine Kinase Inhibitor. Eur J Drug Metab Pharmacokinet 47, 579–592 (2022). https://doi.org/10.1007/s13318-022-00776-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00776-7