Abstract
Background and Objectives
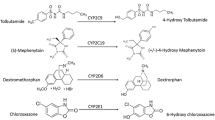
AT-533 is a novel heat shock protein 90 inhibitor, which exhibits various biological activities in vitro and in vivo. Cytochrome P450 (CYP) enzymes in the liver are involved in the biotransformation of drugs and considered to be essential indicators of liver toxicity. The aim of this study was to assess the effect of AT-533, either as active pharmaceutical ingredient or in gel form, on liver CYP enzymes.
Methods
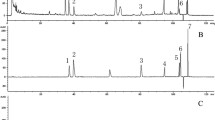
The effect of AT-533 or AT-533 gel on rat liver cytochrome P450 enzymes, including CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4, was analyzed using LC-MS/MS.
Results
AT-533 and AT-533 gel did not significantly increase or reduce the enzymatic activity of CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 at any treatment dose.
Conclusions
AT-533 and AT-533 gel did not have any effect on CYP activity and may be considered safe for external use in gel form, as an alternative to conventional treatment.




Similar content being viewed by others
References
Pinto N, Dolan ME. Clinically relevant genetic variations in drug metabolizing enzymes. Curr Drug Metab. 2011;12(5):487–97. https://doi.org/10.2174/138920011795495321.
Xu S-F, Hu A-L, Xie L, Liu J-J, Wu Q, Liu J. Age-associated changes of cytochrome P450 and related phase-2 gene/proteins in livers of rats. PeerJ. 2019;7: e7429. https://doi.org/10.7717/peerj.7429.
Daskalopoulos EP, Lang MA, Marselos M, Malliou F, Konstandi M. D-2-dopaminergic receptor-linked pathways: critical regulators of CYP3A, CYP2C, and CYP2D. Mol Pharmacol. 2012;82(4):668–78. https://doi.org/10.1124/mol.112.078709.
Kot M, Daujat-Chavanieu M. Altered cytokine profile under control of the serotonergic system determines the regulation of CYP2C11 and CYP3A isoforms (vol 116, pg 369, 2018). Food Chem Toxicol. 2018;118:471–2. https://doi.org/10.1016/j.fct.2018.05.028.
Park SY, Kim CH, Lee JY, Jeon JS, Kim MJ, Chae SH, et al. Hepatic expression of cytochrome P450 in Zucker diabetic fatty rats. Food Chem Toxicol. 2016;96:244–53. https://doi.org/10.1016/j.fct.2016.08.010.
Pathania S, Bhatia R, Baldi A, Singh R, Rawal RK. Drug metabolizing enzymes and their inhibitors’ role in cancer resistance. Biomed Pharmacother Biomed Pharmacothe. 2018;105:53–65. https://doi.org/10.1016/j.biopha.2018.05.117.
Kaur G, Gupta SK, Singh P, Ali V, Kumar V, Verma M. Drug-metabolizing enzymes: role in drug resistance in cancer. Clin Transl Oncol. 2020;22(10):1667–80. https://doi.org/10.1007/s12094-020-02325-7.
Rendic S, Guengerich FP. Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem Res Toxicol. 2015;28(1):38–42. https://doi.org/10.1021/tx500444e.
Sychev DA, Ashraf GM, Svistunov AA, Maksimov ML, Tarasov VV, Chubarev VN, et al. The cytochrome P450 isoenzyme and some new opportunities for the prediction of negative drug interaction in vivo. Drug Des Dev Ther. 2018;12:1147–56. https://doi.org/10.2147/DDDT.S149069.
Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discovery. 2005;4(10):825–33. https://doi.org/10.1038/nrd1851.
Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, et al. Drug-drug interactions for UDP-glucuronosyltransferase substrates: A pharmacokinetic explanation for typically observed low exposure (AUC(i)/AUC) ratios. Drug Metab Dispos. 2004;32(11):1201–8. https://doi.org/10.1124/dmd.104.000794.
Thunell S, Pomp E, Brun A. Guide to drug porphyrogenicity prediction and drug prescription in the acute porphyrias. Br J Clin Pharmacol. 2007;64(5):668–79. https://doi.org/10.1111/j.0306-5251.2007.02955.x.
Vasanthanathan P, Taboureau O, Oostenbrink C, Vermeulen NPE, Olsen L, Jørgensen FS. Classification of cytochrome P450 1A2 inhibitors and noninhibitors by machine learning techniques. Drug Metab Dispos. 2009;37(3):658–64. https://doi.org/10.1124/dmd.108.023507.
Zhou S-F, Yang L-P, Zhou Z-W, Liu Y-H, Chan E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481–94. https://doi.org/10.1208/s12248-009-9127-y.
Vaghela M, Sahu N, Kharkar P, Pandita N. In vivo pharmacokinetic interaction by ethanolic extract of Gymnema sylvestre with CYP2C9 (Tolbutamide), CYP3A4 (Amlodipine) and CYP1A2 (Phenacetin) in rats. Chem-Biol Interact. 2017;278:141–51. https://doi.org/10.1016/j.cbi.2017.10.015.
Van Booven D, Marsh S, McLeod H, Carrillo MW, Sangkuhl K, Klein TE, et al. Cytochrome P450 2C9-CYP2C9. Pharmacogenet Genomics. 2010;20(4):277–81. https://doi.org/10.1097/FPC.0b013e3283349e84.
Krasniqi V, Dimovski A, Domjanović IK, Bilić I, Božina N. How polymorphisms of the cytochrome P450 genes affect ibuprofen and diclofenac metabolism and toxicity. Arh Hig Rada Toksikol. 2016;67(1):1–8. https://doi.org/10.1515/aiht-2016-67-2754.
Baumann P, Eap CB, Gastpar M. The effect of perazine on the CYP2D6 and CYP2C19 phenotypes as measured by the dextromethorphan and mephenytoin tests in psychiatric patients. Basic Clin Pharmacol. 2020;126(5):444–7. https://doi.org/10.1111/bcpt.13373.
Wang Y, Wang C, Wang S, Zhou Q, Dai D, Shi J, et al. Cytochrome P450-based drug-drug interactions of vonoprazan and. Front Pharmacol. 2020;11:53. https://doi.org/10.3389/fphar.2020.00053.
Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–41. https://doi.org/10.1016/j.pharmthera.2012.12.007.
Šarić Mustapić D, Debeljak Ž, Maleš Ž, Bojić M. The inhibitory effect of flavonoid aglycones on the metabolic activity of CYP3A4 enzyme. Molecules (Basel, Switzerland). 2018. https://doi.org/10.3390/molecules23102553.
Xiao K, Gao J, Weng S-J, Fang Y, Gao N, Wen Q, et al. CYP3A4/5 activity probed with testosterone and midazolam: correlation between two substrates at the microsomal and enzyme levels. Mol Pharmaceut. 2019;16(1):382–92. https://doi.org/10.1021/acs.molpharmaceut.8b01043.
Wang SX, Wang X, Du Z, Liu YT, Huang DN, Zheng K, et al. SNX-25a, a novel Hsp90 inhibitor, inhibited human cancer growth more potently than 17-AAG. Biochem Bioph Res Co. 2014;450(1):73–80. https://doi.org/10.1016/j.bbrc.2014.05.076.
Zhang P-C, Liu X, Li M-M, Ma Y-Y, Sun H-T, Tian X-Y, et al. AT-533, a novel Hsp90 inhibitor, inhibits breast cancer growth and HIF-1α/VEGF/VEGFR-2-mediated angiogenesis in vitro and in vivo. Biochem Pharmacol. 2020;172: 113771. https://doi.org/10.1016/j.bcp.2019.113771.
Li F, Jin F, Wang Y, Zheng D, Liu J, Zhang Z, et al. Hsp90 inhibitor AT-533 blocks HSV-1 nuclear egress and assembly. J Biochem. 2018;164(6):397–406. https://doi.org/10.1093/jb/mvy066.
Wang Y, Wang R, Li F, Wang Y, Zhang Z, Wang Q, et al. Heat-shock protein 90alpha is involved in maintaining the stability of VP16 and VP16-mediated transactivation of alpha genes from herpes simplex virus-1. Mol Med. 2018;24(1):65. https://doi.org/10.1186/s10020-018-0066-x.
Xiang YF, Qian CW, Xing GW, Hao J, Xia M, Wang YF. Anti-herpes simplex virus efficacies of 2-aminobenzamide derivatives as novel HSP90 inhibitors. Bioorg Med Chem Lett. 2012;22(14):4703–6. https://doi.org/10.1016/j.bmcl.2012.05.079.
Li F, Song X, Su G, Wang Y, Wang Z, Qing S, et al. AT-533, a Hsp90 inhibitor, attenuates HSV-1-induced inflammation. Biochem Pharmacol. 2019;166:82–92. https://doi.org/10.1016/j.bcp.2019.05.003.
Zeng Y. The evaluation of the safety and lead accumulation of Gou-pi plaster (a traditional external preparation) [PhD dissertation]: Chengdu University of TCM; 2012.
Renwick AB, Lavignette G, Worboys PD, Williams B, Surry D, Lewis DFV, et al. Evaluation of 7-benzyloxy-4-trifluoromethylcoumarin, some other 7-hydroxy-4-trifluoromethylcoumarin derivatives and 7-benzyloxyquinoline as fluorescent substrates for rat hepatic cytochrome P450 enzymes. Xenobiotica. 2001;31(12):861–78. https://doi.org/10.1080/00498250110074063.
Krogstad V, Peric A, Robertsen I, Kringen MK, Vistnes M, Hjelmesæth J, et al. Correlation of body weight and composition with hepatic activities of cytochrome P450 enzymes. J Pharm Sci. 2021;110(1):432–7. https://doi.org/10.1016/j.xphs.2020.10.027.
Brill MJE, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CAJ. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304. https://doi.org/10.2165/11599410-000000000-00000.
Zhong D-F, Zhang S-Q, Sun L, Zhao X-Y. Metabolism of roxithromycin in phenobarbital-treated rat liver microsomes. Acta Pharmacol Sin. 2002;23(5):455–60.
Handschin C, Meyer UA. Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev. 2003;55(4):649–73. https://doi.org/10.1124/pr.55.4.2.
Bojić M, Kondža M, Rimac H, Benković G, Maleš Ž. The Effect of Flavonoid Aglycones on the CYP1A2, CYP2A6, CYP2C8 and CYP2D6 Enzymes Activity. Molecules (Basel, Switzerland). 2019. https://doi.org/10.3390/molecules24173174.
Glowacki LL, Hodges LD, Wynne PM, Wright PFA, Kalafatis N, Macrides TA. LC-MSMS characterisations of scymnol and oxoscymnol biotransformations in incubation mixtures of rat liver microsomes. Biochimie. 2019;160:130–40. https://doi.org/10.1016/j.biochi.2019.02.016.
Acknowledgments
Yanting Wu would like to thank Mr. Hui Chen (Guangzhou University of Chinese Medicine) for the care and encouragement of the experimental progress.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Guangzhou Major Program of the Industry-University-Research collaborative innovation (Grant numbers: 201604020178 & 201704030087).
Conflicts of interest
The authors declare no conflict of interest.
Ethics Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed and the study protocols were approved by The Institutional Animal Care and Use Committee of Jinan University Guangzhou, PR China.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All authors confirm that all data and materials as well as software application or custom code support their published claims and comply with field standards.
Code availability
Not applicable.
Authors' contributions
YW designed the study. YW, ML, YG, TL, LZ, CH, CY, QL and ZR performed the experiments. YW and ML performed the statistical analysis. YW drafted the manuscript. ML and YG made significant conceptual contributions to the manuscript. ZR and YW reviewed the final version of the paper. All the authors provided intellectual content and approved the final version of the manuscript.
Rights and permissions
About this article
Cite this article
Wu, Y., Li, M., Guo, Y. et al. The Effects of AT-533 and AT-533 gel on Liver Cytochrome P450 Enzymes in Rats. Eur J Drug Metab Pharmacokinet 47, 345–352 (2022). https://doi.org/10.1007/s13318-022-00757-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00757-w