Abstract
The health burden of ischemic stroke is high and will continue to increase with an aging population. Recurrent ischemic stroke is increasingly recognized as a major public health concern with potentially debilitating sequelae. Thus, it is imperative to develop and implement effective strategies for stroke prevention. When considering secondary ischemic stroke prevention, it is important to consider the mechanism of the first stroke and the related vascular risk factors. Secondary ischemic stroke prevention typically includes multiple medical and, potentially, surgical treatments, but with the shared goal of reducing the risk of recurrent ischemic stroke. Providers, health care systems, and insurers also need to consider the availability of treatments, their cost and patient burden, methods for improving adherence, and interventions that target lifestyle risk factors such as diet or activity. In this article, we discuss aspects from the 2021 AHA Guideline on Secondary Stroke Prevention as well as highlight additional information relevant to best practices for reducing recurrent stroke risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the second leading cause of death worldwide, and it is characterized by high morbidity. Approximately 50% of stroke survivors are chronically disabled, and, thus, the public health burden of stroke is immense [1]. Ischemic stroke occurs when the blood flow to an area of the brain is restricted or blocked as a result of stenosis or occlusion of an artery either in the neck or brain. Hemorrhagic stroke, in contrast, is the result of rupture of a blood vessel and bleeding into the brain or on the brain’s surface [2]. Because hemorrhagic stroke has distinct mechanisms of disease and prevention strategies, the focus of this article will be ischemic stroke, which is subsequently referred to as “stroke.”
The risk of stroke doubles every ten years after 55 years of age. With an aging population, the prevalence of stroke will continue to increase over the next two decades [3]. One quarter of the ~ 700,000 ischemic strokes a year in the USA are recurrent strokes [4, 5]. Patients who have suffered a recurrent stroke are twice as likely to die and have worse functional outcome compared to patients with a first-ever stroke [6,7,8,9]. In addition, the hospitalization cost is twice as high for recurrent stroke compared to first ever stroke [10]. Although in this review we delineate the terms stroke and TIA separately, it is important to note that the utility of the diagnosis of TIA in this context has come under question, suggesting that the entities are equivalent when considering risk of recurrence and secondary prevention [11].
Secondary stroke prevention is distinct from primary stroke prevention because it necessitates attention to the clinical features of the initial stroke, such as the type of stroke, the mechanism, and the recognition of any contributing medical comorbidities [12]. Prevention approaches begin with identifying the most likely possible mechanism of the first stroke and optimizing the associated modifiable risk factors. The management of these risk factors is a multifaceted process and typically includes both lifestyle modifications, such as increasing aerobic activity and eating a more nutritious diet, as well as the administration of lipid lowering, blood pressure lowering, and antithrombotic medications [13]. Stroke prevention can be reasonably achieved by addressing modifiable risk factors [14]. Unfortunately, these risk factors are poorly controlled for the majority of the population and typically remain poorly controlled in many who have suffered a primary stroke [15].
Diagnostic Evaluation
After the identification of a stroke or TIA, patients require a diagnostic evaluation to understand the mechanism of the stroke, ideally within 48 h of the suspected onset of the event [16]. The goal of this diagnostic evaluation is to tailor treatments to the patient to lower the risk of recurrent stroke. Providers should perform a CT or an MRI of the brain to ensure that the diagnosis of stroke is substantiated [17]. A laboratory workup is important, including a complete blood count, prothrombin time, partial thromboplastin time, random or fasting glucose level, HbA1c, kidney function tests, and a fasting lipid profile. These lab results can provide insights into risk factors that warrant treatment to lower the risk of recurrent stroke [18]. Additionally, providers should perform an ECG to screen for cardiac arrhythmias, primarily atrial fibrillation, and a transthoracic echocardiogram to evaluate for valvular abnormalities, intracardiac thrombus, or patent foramen ovale (indicated in individuals aged ≤ 60) [19, 20].
A typical stroke workup also includes noninvasive angiography with CT angiography or magnetic resonance angiography, although ultrasound of the arteries in the neck and brain is a potential alternative. Digital subtraction angiography is usually reserved for situations where noninvasive studies are inconclusive. Angiographic tests allow for the identification of potential arterial stenosis, thromboses, dissection, or other vasculopathies [21]. In selected patients, who lack a clear diagnosis at the end of this testing, or those designated to have an embolic stroke of undetermined source (ESUS), extended cardiac monitoring for weeks to years may be indicated, to increase the yield of atrial fibrillation diagnosis [20, 22, 23].
Major Risk Factors
The major risk factors for stroke are delineated into two subcategories, those that are modifiable and those that are not modifiable [24]. Note that when evidence from a randomized clinical trial (RCT) is provided, this is accompanied by the inclusion of an RCT identifier.
Modifiable Risk Factors
Among the modifiable risk factors are hypertension, hyperlipidemia, diabetes mellitus, smoking, physical inactivity, poor diet, and obesity. Hypertension is typically regarded as the most important of the modifiable risk factors for stroke [25]. Hypertension causes recurrent stroke through mechanisms that span every organ system and stroke etiology [26]. Hyperlipidemia, which can be both an acquired or a genetic trait, is associated with higher risk of ischemic stroke [27]. An increased level of cholesterol in the blood can lead to the buildup of atherosclerosis in arteries, which can cause stroke either through reduced blood flow, occlusive disease, or the formation of thromboemboli.
Another major risk factor for stroke is diabetes mellitus, both types 1 and 2 [28]. The morbidity of diabetes occurs through both macrovascular and microvascular disease, which respectively refers to large vessel atherosclerosis and the complications of neuropathy, nephropathy, retinopathy, and chronic microvascular disease of the brain including lacunar infarctions [29]. For the vast majority of patients, particularly those < 65 years of age, it is recommended to achieve a hemoglobin A1c (HbA1c) that is less than 7% [30]. Diabetes is associated with a twofold higher risk of stroke [31], and there is a U-shaped association of HbA1c and stroke risk, which is further pronounced for patients that are using antidiabetic, antihypertensive, or lipid-lowering medications [32]. Diabetics can reduce the risk of recurrent stroke through both medication usage and the implementation of behavioral practices that lead to better glucose control.
Active smoking causes atherosclerosis, raises blood pressure, and can trigger episodes of atrial fibrillation, all of which increase the likelihood of experiencing a stroke [33]. For patients who have experienced a stroke or TIA that continue to smoke tobacco, it is recommended that they seek counseling, which may or may not include drug therapy, to aid in quitting smoking [34]. Similarly, patients who have experienced a primary stroke or TIA that consume more than two alcoholic beverages daily for men or more than one for women should seek to reduce their consumption of alcohol to limit the risk of experiencing a second stroke [35].
Physical inactivity is also associated with an increased risk of stroke through a variety of mechanisms. In general, being sedentary leads to an overall decline in health that can indirectly contribute to experiencing a stroke [25]. Conversely, increased physical activity lowers the risk of cardiac events and other vascular issues such as stroke [36]. A study performed by the Cooper Clinic assessed the relationship between cardiovascular fitness and stroke mortality in healthy men aged 40–87 with up to a decade of follow-up. They discovered an inverse relationship such that those in the highest activity group were determined to experience a 68% lower risk of stroke and subsequent death than those in the lowest fitness group [37].
Specifically for the prevention of recurrent stroke, patients that remain capable of performing physical activity after their first stroke or TIA should engage in moderate intensity aerobic activity for a minimum of 10 min, four times a week, or they can choose to engage in vigorous-intensity aerobic activity for a minimum of 20 min, twice a week [38]. Furthermore, it has been shown that performing physical activity post-stroke leads to improvements in cognitive function, including a general improvement in cognitive performance (Hedges’ g [95% CI] = 0.304 [0.14–0.47]) and improvements in attention and processing speed (Hedges’ g [95% CI] = 0.37 [0.10–0.63]). The greatest cognitive gains have been shown to result from exercise regimens that include both aerobic and strength training (Hedges’ g [95% CI] = 0.43 [0.09, 0.77]) [39].
While performing physical activity has numerous benefits of its own, pairing this activity with a balanced and healthy diet leads to further improvements in health. Following a healthy diet is associated with the prevention of vascular events, and balanced nutritional plans such as the Mediterranean diet help in the prevention and treatment of atherosclerotic cardiovascular disease (ASCVD) [40]. A high adherence to this diet has been shown to reduce the risk of cerebrovascular events [relative risk (RR) 0.71, 95% CI 0.57–0.89] [41]. This diet emphasizes the consumption of monounsaturated fats, primarily fish, extra virgin olive oils, and plant-based foods such as nuts [42]. Increasing the consumption of fruits and vegetables has also been shown to be beneficial in preventing stroke [relative risk reduction (RRR) 0.79, 95% CI 0.71–0.84] [43]. Additionally, for hypertensive individuals who have suffered a stroke or TIA, restricting the intake of dietary sodium by approximately a gram a day has been shown to be beneficial in preventing recurrent stroke [44]. Specifically, individuals who consume ≥ 4 g of sodium per day display an increased risk of stroke (HR = 2.59; 95% CI = 1.27–5.28) than those who consume ≤ 1.5 g/day [45].
A final major modifiable risk factor for stroke is obesity or high body mass index (BMI). Obesity is a complex pathology that has both genetic and lifestyle components. By using medication and lifestyle modification to control obesity, patients can reduce their risk of stroke and ASCVD [46]. The reduction in weight helps indirectly, by improving risk factors including blood pressure and cholesterol levels [21]. More recently metabolic health in concert with BMI is being investigated in relation to stroke risk and cardiovascular disease [47]. In general, metabolically unhealthy obese individuals have an increased risk of ischemic stroke (hazard ratio, 1.30 [95% CI, 1.09–1.56]), compared to metabolically healthy participants with a normal BMI [48]. The current literature supports that for patients who have had a stroke or TIA and are obese, it is recommended that they undergo a comprehensive behavioral lifestyle modification program to achieve and sustain significant weight loss and improve fitness [49].
These modifiable risk factors, their importance, and treatments are summarized in Table 1.
Non-modifiable Risk Factors
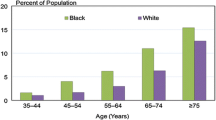
Among the non-modifiable risk factors for stroke is age. Advanced age remains the most significant risk factor for stroke [50]. Additionally, there are racial and ethnic factors that predispose an individual to stroke. Black patients have a higher risk of stroke than White patients [51]. This increased risk is shown to be due to the influence of social determinants of health and well-being rather than inherent biology [52]. The sex of an individual also affects their risk of stroke, where for the vast majority of ages, men have a higher risk of experiencing a stroke than women [53]. However, because women live longer than men, the lifetime rate of stroke for women is higher. Family history of stroke, heart disease, and any other heritable vascular disease are also a risk factor of stroke [54]. Although stroke prevention does not particularly focus on these risk factors as they are non-modifiable, they are important considerations in the assessment of stroke risk and are potential considerations in shaping the treatment plan.
When considering secondary stroke prevention, patients who had minimal risk factor management prior to the first stroke warrant a stronger emphasis on managing risk factors and potentially more medical follow-up and exposure to healthcare. However, if an individual has few risk factors or the risk factors are well managed prior to the experience of a first stroke, then a broader approach towards considering all potential risk factors is necessitated [55].
Medical Treatment
Antithrombotic Therapy
Antithrombotic therapy is the use of antiplatelet and/or anticoagulant medications to block the formation of clots. Considering the administration of these agents on a short- or long-term course is an important step in the secondary prevention of stroke [21]. For non-cardioembolic stroke, antiplatelet therapy is recommended. Although 3 weeks to 3 months of dual anti-platelet therapy (aspirin combined with clopidogrel) is beneficial in mild stroke and high-risk TIA [56] or symptomatic intracranial atherosclerosis [57], trials of longer term dual antiplatelet therapy for secondary stroke prevention have consistently shown either no benefit or harm [58,59,60]. For these reasons, long-term antiplatelet monotherapy is the treatment of choice for secondary stroke prevention in patients with non-cardioembolic stroke.
Aspirin, aspirin-extended release dipyridamole, or clopidogrel are all acceptable choices. In the study by Antiplatelet Trialists’ Collaboration, aspirin as an agent of long-term antiplatelet monotherapy decreased the risk of subsequent vascular event by 22% [61]. Also, in a Cochrane systematic review of eight RCTs, 160–300 mg of aspirin daily initiated within 48 h of stroke onset reduced the risk of recurrent stroke without significantly increasing the risk of hemorrhagic complications. For every 1000 people treated with aspirin, seven people would avoid recurrent stroke [61, 62]. The European Stroke Prevention Study (ESPS) RCT randomly assigned patients with either stroke or TIA to aspirin/dipyridamole (325 mg/75 mg) or placebo three times a day. The aspirin/dipyridamole group showed a 33% relative risk reduction in stroke recurrence and death [63]. Because aspirin/dipyridamole causes more side effects than aspirin, most providers use aspirin monotherapy instead.
The clopidogrel vs. aspirin in patients at risk of ischemic events RCT reported that clopidogrel 75 mg daily did not differ from aspirin 325 mg in terms of relative risk reduction of vascular events for patients with prior stroke [64]. For other antiplatelet monotherapies, ticagrelor has not shown superior benefit or a better safety profile compared to aspirin in any of the available RCTs, and has a higher rate of discontinuation due to dyspnea or bleeding [65]. The CSPS RCT showed that cilostazol 100 mg two times daily vs. placebo is associated with a relative stroke risk reduction of 41.7% [66]. In the CSPS-2 RCT, at mean of 29 month follow up, cilostazol 100 mg two times daily compared to aspirin 81 mg daily had 34% relative risk reduction in stroke with a much lower frequency of hemorrhagic events (0.77% vs. 1.78%, p = 0.0004) [67]. However, most cilostazol trials were conducted in East Asian patients, and thus, these results need further validation to be generalizable globally [68].
In the COMPASS RCT, low-dose rivaroxaban 2.5 mg twice a day plus aspirin 75 mg was compared with aspirin 75 mg daily in patients with stable atherosclerotic vascular disease without atrial fibrillation (AF). The rate of stroke recurrence was 4.1% in the combination therapy group vs. 5.4% in the aspirin alone group. However major bleeding was 3.1% vs. 1.9%, respectively [69]. For ESUS, dabigatran in the RE-SPECT ESUS trial and rivaroxaban in the NAVIGATE ESUS RCTs conferred a higher risk of bleeding, and neither medication was superior to aspirin in secondary stroke prevention [70, 71]. The AVERROES RCT did not show a higher risk of bleeding when comparing low dose apixaban (2.5 mg twice daily) to aspirin, but that trial enrolled individuals with atrial fibrillation, not ESUS, and only 14% of subjects had a prior history of stroke [72, 73]. Therefore, anti-platelet therapy remains the primary choice for ESUS.
Anticoagulation for stroke prevention is reserved for patients with proven AF, known cardiac or arterial thrombus, mechanical heart valves, or selected hypercoagulable disorders. AF sometimes exists before the first stroke and may also develop or be identified afterwards and lead to a higher risk for recurrent stroke [21]. For patients that have non-valvular or valvular AF and have had a stroke or TIA, it is recommended that they receive oral anticoagulation medications to best reduce the risk of recurrent stroke [74, 75]. For non-valvular AF, oral anticoagulation is recommended regardless of the pattern of AF [76]. For patients with a left ventricular or atrial thrombus identified during their stroke workup, it is recommended that they be anticoagulated for a duration of at least 3 months to reduce the chance of recurrent stroke [77].
The available anticoagulants for stroke prevention include warfarin and direct oral anticoagulants (DOACs). Warfarin has been a mainstay of treatment for cardioembolic stroke and hypercoagulable conditions for many years. However, DOACs have replaced warfarin in many non-valvular AF patients [78]. DOACs include rivaroxaban, apixaban, edoxaban, and dabigatran. DOACs have similar or superior efficacy in preventing strokes with reduced or similar intracranial bleeding risk compared to warfarin. The RE-LY RCT compared dabigatran with warfarin with the primary end point of stroke recurrence and systemic embolization in patients with non-valvular AF. Dabigatran was modestly superior to warfarin at 150 mg twice a day (1.11% vs. 1.69%), and non-inferior to warfarin at 110 mg twice a day (1.53% vs. 1.69%) [79]. In the AVERROES RCT, apixaban showed similar modest risk reduction compared to warfarin. Recurrence of stroke and systemic embolization was 1.27% for apixaban vs. 1.67% for warfarin [80]. However, in patients with prior history of stroke or TIA, there was no significant difference between apixaban and warfarin with regard to stroke or systemic embolization recurrence. Also, rivaroxaban was not inferior to warfarin in non-valvular AF patients in the ROCKET-AF RCT [81]. Additionally, edoxaban has shown non-inferiority in both low (30 mg once daily) and high (60 mg once daily) doses compared to warfarin in patients with non-valvular AF [82].
Antiypertensive Therapy
Hypertension is the leading modifiable risk factor for stroke with more than 50% of the global burden of stroke attributable to hypertension [83]. RCTs on antihypertensive medication have shown that there is significant decrease in first ever stroke with reduction in blood pressure [84]. Meta-analyses of RCTs have also demonstrated that lowering blood pressure would reduce the risk of stroke recurrence by 20–30% [85], but long-term blood pressure control after stroke remains poor, primarily due to undertreatment or under-adherence [86]. There is still uncertainty regarding the timing of blood pressure lowering after stroke or TIA, partly due to concerns of having a negative effect on cerebral perfusion as well as a scarcity of large RCTs on this subject [87].
A limited number of RCTs have evaluated intensive blood pressure lowering (systolic blood pressure < 130 mm Hg) vs. other less aggressive blood pressure targets. All these RCTs reported non-significant reduced risk of stroke recurrence with intensive blood pressure control [26, 88,89,90]. However, most clinical guidelines stipulate a BP target of 130/80 mm Hg or lower for long-term secondary stroke prevention, at least for patients with previous hypertension. The justification for this recommendation has a strong basis in the benefits of hypertension control for other organ systems including the heart and kidneys [91].
Five classes of hypertension medication have been evaluated for secondary stroke prevention, including β-adrenergic antagonists, calcium channel antagonists, diuretics, angiotensin-converting enzyme (ACE) inhibitor, and angiotensin II receptor blockers (ARBs). The majority of available RCTs have evaluated single therapy versus placebo, and therefore, no direct comparison was made between medication classes [92,93,94,95]. A recent meta-analysis of fourteen RCTs included 42,736 patients, of which two-thirds were from the PROGRESS, PRoFESS, or SPS3 trials, demonstrated that antihypertensive therapy was associated with lower stroke recurrence (RR 0.73, 95% CI 0.62–0.87) with a favorable trend that did not reach significance for incident ischemic stroke (RR 0.87; 95% CI 0.70–1.07) [85].
A meta-analysis of secondary prevention stroke RCTs estimated comparative effectiveness of antihypertensive medications. The authors concluded that a diuretic-based therapy was possibly superior to other therapies [96]. Calcium channel antagonists have not been evaluated in large randomized trials of secondary stroke prevention. However, they have a similar effect on BP reduction compared to ACE inhibitors in primary stroke prevention and smaller secondary prevention studies [96, 97]. Nonetheless, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers are considered the first line agents in preference to calcium channel blockers or beta blockers [98, 99].
Hyperlipidemia Therapy
Managing hyperlipidemia is another important aspect of secondary stroke prevention. Specifically, statin therapy is proven to lower the risk of stroke recurrence.
The heart protection study (HPS) was the first large-scale RCT of simvastatin to include patients with cerebrovascular disease. In the group of patients with previous stroke, treatment with simvastatin had no effect on stroke recurrence rate but was associated with a significant reduction of major vascular events by 20% (24.7% vs. 29.8%) irrespective of the stroke subtype [100]. Two RCTs, J-STARS and SPARCL, studied statins in non-cardioembolic stroke patients and showed significant reduction in stroke recurrence. In J-STARS, non-cardioembolic stroke patients were randomized to low dose pravastatin or placebo [101]. Patients treated with pravastatin had a significant reduction in recurrence rate of atherothrombotic stroke (0.21% for pravastatin vs. 0.64% for placebo). In the SPARCL RCT of patients with prior non-cardioembolic stroke, treatment with atorvastatin 80 mg daily had an absolute risk reduction of 2.2% for stroke but patients had a significant increase (HR 1.66, 95% CI 1.08–2.55) in the risk of ICH [102]. An exploratory analysis of SPARCL showed that high-dose atorvastatin was similarly efficacious in preventing strokes irrespective of baseline ischemic stroke subtype. Also, a recent meta-analysis on statin therapy for secondary stroke prevention showed that among 10,394 patients with prior stroke, statin treatment was associated with an absolute risk reduction of 1.6% for stroke recurrence [103].
Therefore for patients who have experienced an ischemic stroke and have an LDL cholesterol greater than 100 mg/dL, it is recommended to start a high intensity statin such as 80 mg of atorvastatin or 20–40 mg of rosuvastatin to reduce the risk of secondary stroke [104]. To ensure that hyperlipidemia is being appropriately managed, it is recommended that patients obtain measurements of their LDL-C levels 4 weeks after beginning a statin and then continue to measure levels every 3 to 6 months thereafter. Ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (evolocumab, alirocumab) have been shown to reduce the risk of cardiovascular events and can be used as an alternative in patients who cannot tolerate statins or do not have an adequate response, typically defined as an LDL-C < 70 while on therapy [51]. However, prior to considering administration of a PCSK9 inhibitor, it may be worth adding ezetimibe to the current statin treatment and assessing if LDL-C lowers to < 70 [105].
The IMPROVE-IT RCT showed that the addition of ezetimibe to standard simvastatin treatment in patients with prior stroke would result in a reduced risk of ischemic stroke. Subgroup analyses of the FOURIER and ODYSSEY OUTCOMES RCTs involving patients with a prior history of stroke confirmed a benefit of PCSK9 inhibitors in the reduction of cardiovascular events and a numerical decrease in the rate of stroke recurrence [106, 107]. Total stroke events among patients with prior stroke were non-significantly reduced with evolocumab vs. placebo (RR 0.87, 95% CI 0.65–1.16) [106].
Hyperglycemia Therapy
For patients who have had an ischemic stroke or TIA and have type 2 diabetes (T2DM), the goal HgbA1c is < 7. Oral or injectable hypoglycemic drugs and insulin are proven methods to achieve glycemic control. Of the many medication classes for treating T2DM, thiazolidinedione (TZD) and glucagon-like-1 (GLP1) receptor agonists have shown some benefit for secondary stroke prevention. A Cochrane review comparing TZDs and placebo for secondary stroke prevention identified four RCTs with 1163 participants and reported that TZDs are associated with reduced stroke recurrence (RR 0.52, 95% CI 0.34 to 0.80) [108].
A meta-analysis of RCTs, including 56,004 participants, on the effects of GLP1s on major adverse cardiovascular events, demonstrated a significant reduction in cardiovascular events and stroke. GLP-1 s were also associated with a significant reduction in fatal and non-fatal stroke (HR: 0.84; 95% CI 0.76–0.94) [109]. If patients have difficulty managing their glucose levels, it is recommended that they establish care in a multimodal diabetes clinic and seek nutritional and lifestyle therapy and obtain a basic education on self-managing glucose level.
Carotid Stenosis Therapy
If extracranial carotid artery stenosis is identified, it can be treated by carotid endarterectomy or carotid artery stenting [8]. For patients who have had a TIA or a nondisabling ischemic stroke within the past 6 months that have severe (≥ 70% stenosis) ipsilateral carotid artery stenosis, carotid revascularization decreases the risk of future stroke by 16%. However, when the stenosis severity is 50–69% the recommendation to revascularize is strongest when it is determined that the risk of perioperative morbidity and mortality is less than 6% [110]. Equally important are patient-specific factors in the decision making, as the decision to undergo an invasive procedure is complex and personal. For patients who elect to undergo carotid revascularization, the procedure should be performed by surgeons with expertise in placing a stent or performing endarterectomy to meet the risk assessment of less than 6% [111]. Additionally, patients should be receiving intensive medical therapy coupled with antiplatelet and lipid-lowering therapy combined with an aggressive antihypertensive regimen to offer the best chance at preventing recurrent stroke [104].
Investigational Therapies
There are a variety of drugs under investigation for secondary stroke prevention, primarily in the antithrombotic class. For example cilostazol, which is a phosphodiesterase 3 inhibitor, remains under investigation for potential use in preventing secondary stroke [112, 113]. Two pharmaceutical companies are conducting large multinational RCTs of oral factor XIa inhibitors as add-on to antithrombotic therapy in patients with non-cardioembolic stroke and high-risk TIA. However, the recently published phase 2b study of Bayer’s factor XIa inhibitor for stroke prevention (PACIFIC-Stroke, n = 1808) failed to show a benefit for secondary stroke prevention, but may have been underpowered [81]. Additionally, there are emerging lipid-lowering agents for stroke prevention that include oral small molecules (bempedoic acid, oral inhibitor of ATP citrate lyase), monoclonal antibodies (evinacumab targeting angiopoietin like protein 3), and various ribonucleic acid (RNA) knockdown strategies (inclisiran, siRNA-targeting PCSK9; AROANG3, siRNA-targeting angiopoietin like protein 3; olpasiran, siRNA-targeting LPA). While these agents may be proven to have benefit for secondary stroke prevention, considerations of cost, side effects, and effectiveness will need to be evaluated prior to widespread adoption.
Conclusion
The management of both vascular and lifestyle risk factors remains central to the prevention of secondary stroke. Ischemic stroke etiology is important in developing patient-specific recommendations for prevention of recurrent stroke, and as such performing a proper diagnostic workup of the primary stroke is essential. Approaching management of risk factors from a multidisciplinary perspective that includes medical, surgical, behavioral, and self-management education offers the best chance of success.
Many of the changes an individual must make after experiencing a stroke or TIA are behavioral. As such, it is necessary that patients be provided with opportunities to improve knowledge of their medical conditions and personalized methods to ensure maximal treatment adherence. Only through these methods can providers ensure that patients are well apprised of their condition, risk factors, and what they can do to prevent recurrent stroke.
References
Donkor ES. Stroke in the 21st century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;27(2018):3238165.
Hui C, Tadi P, Patti L. Ischemic stroke. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2022 Oct 23]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK499997/.
Béjot Y, Bailly H, Graber M, Garnier L, Laville A, Dubourget L, et al. Impact of the ageing population on the burden of stroke: the Dijon stroke registry. Neuroepidemiology. 2019;52(1–2):78–85.
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-528.
Oza R, Rundell K, Garcellano M. Recurrent ischemic stroke: strategies for prevention. Am Fam Physician. 2017;96(7):436–40.
Skajaa N, Adelborg K, Horváth-Puhó E, Rothman KJ, Henderson VW, Thygesen LC, et al. Risks of stroke recurrence and mortality after first and recurrent strokes in Denmark: a nationwide registry study. Neurology. 2022;98(4):e329–42.
Khanevski AN, Bjerkreim AT, Novotny V, Næss H, Thomassen L, Logallo N, et al. Recurrent ischemic stroke: incidence, predictors, and impact on mortality. Acta Neurol Scand. 2019;140(1):3–8.
Nedeltchev K, der Maur TA, Georgiadis D, Arnold M, Caso V, Mattle HP, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry. 2005;76(2):191–5.
Serena J, Segura T, Roquer J, García-Gil M, Castillo J. The ARTICO study: identification of patients at high risk of vascular recurrence after a first non-cardioembolic stroke. BMC Neurol. 2015;15:28.
Engel-Nitz NM, Sander SD, Harley C, Rey GG, Shah H. Costs and outcomes of noncardioembolic ischemic stroke in a managed care population. Vasc Health Risk Manag. 2010;6:905–13.
Easton JD, Johnston SC. Time to retire the concept of transient ischemic attack. JAMA. 2022;327(9):813–4.
Moroney JT, Bagiella E, Paik MC, Sacco RL, Desmond DW. Risk factors for early recurrence after ischemic stroke. Stroke. 1998;29(10):2118–24.
Esenwa C, Gutierrez J. Secondary stroke prevention: challenges and solutions. Vasc Health Risk Manag. 2015;7(11):437–50.
Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15(9):913–24.
Razmara A, Ovbiagele B, Markovic D, Towfighi A. Patterns and predictors of blood pressure treatment, control, and outcomes among stroke survivors in the United States. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2016;25(4):857–65.
Arsava EM, Kim GM, Oliveira-Filho J, Gungor L, Noh HJ, de Jesus Lordelo M, et al. Prediction of early recurrence after acute ischemic stroke. JAMA Neurol. 2016;73(4):396–401.
Newman-Toker DE, Moy E, Valente E, Coffey R, Hines AL. Missed diagnosis of stroke in the emergency department: a cross-sectional analysis of a large population-based sample. Diagn Berl Ger. 2014;1(2):155–66.
Roquer J, Rodríguez-Campello A, Cuadrado-Godia E, Giralt-Steinhauer E, Jiménez-Conde J, Soriano C, et al. The role of HbA1c determination in detecting unknown glucose disturbances in ischemic stroke. PLoS ONE. 2014;9(12):e109960.
Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377–87.
Ford B, Peela S, Roberts C. Secondary prevention of ischemic stroke: updated guidelines from AHA/ASA. Am Fam Physician. 2022;105(1):99–102.
Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467.
Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source. Stroke. 2017;48(4):867–72.
Sagris D, Harrison SL, Buckley BJR, Ntaios G, Lip GYH. Long-term cardiac monitoring after embolic stroke of undetermined source: search longer, look harder. Am J Med. 2022;135(9):e311–7.
Boehme AK, Esenwa C, Elkind MSV. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472–95.
O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a casecontrol study. Lancet. 2016;388:761–775.
Kitagawa K, Yamamoto Y, Arima H, Maeda T, Sunami N, Kanzawa T, et al. Effect of standard vs intensive blood pressure control on the risk of recurrent stroke: a randomized clinical trial and meta-analysis. JAMA Neurol. 2019;76(11):1309–18.
Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63(10):1868–75.
Janghorbani M, Hu FB, Willett WC, Li TY, Manson JE, Logroscino G, et al. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes. Diabetes Care. 2007;30(7):1730–5.
Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–35.
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Mitsios JP, Ekinci EI, Mitsios GP, Churilov L, Thijs V. Relationship between glycated hemoglobin and stroke risk: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(11):e007858.
Shen Y, Shi L, Nauman E, Katzmarzyk P, Price-Haywood E, Bazzano A, et al. Association between hemoglobin A1c and stroke risk in patients with type 2 diabetes. J Stroke. 2020;22(1):87–98.
Peters SAE, Huxley RR, Woodward M. Smoking as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 81 cohorts, including 3,980,359 individuals and 42,401 strokes. Stroke. 2013;44:2821–2828.
Chen J, Li S, Zheng K, Wang H, Xie Y, Xu P, et al. Impact of smoking status on stroke recurrence. J Am Heart Assoc. 2019;8(8):e011696.
Patra J, Taylor B, Irving H, Roerecke M, Baliunas D, Mohapatra S, et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types–a systematic review and meta-analysis. BMC Public Health. 2010;18(10):258.
Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–53.
Do Lee C, Blair SN. Cardiorespiratory fitness and stroke mortality in men. Med Sci Sports Exerc. 2002;34(4):592–5.
Turan TN, Nizam A, Lynn MJ, Egan BM, Le NA, Lopes-Virella MF, et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology. 2017;88(4):379–85.
Oberlin LE, Waiwood AM, Cumming TB, Marsland AL, Bernhardt J, Erickson KI. Effects of physical activity on post-stroke cognitive function: a meta-analysis of randomized controlled trials. Stroke. 2017;48(11):3093–100.
English C, MacDonald-Wicks L, Patterson A, Attia J, Hankey GJ. The role of diet in secondary stroke prevention. Lancet Neurol. 2021;20(2):150–60.
Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74(4):580–91.
Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34.
Hu D, Huang J, Wang Y, Zhang D, Qu Y. Fruits and vegetables consumption and risk of stroke: a meta-analysis of prospective cohort studies. Stroke. 2014;45(6):1613–9.
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, DASH-Sodium Collaborative Research Group, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10.
Gardener H, Rundek T, Wright CB, Elkind MSV, Sacco RL. Dietary sodium and risk of stroke in the Northern Manhattan Study. Stroke J Cereb Circ. 2012;43(5):1200–5.
Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673–89.
Quiñones-Ossa GA, Lobo C, Garcia-Ballestas E, Florez WA, Moscote-Salazar LR, Agrawal A. Obesity and stroke: does the paradox apply for stroke? Neurointervention. 2021;16(1):9–19.
Horn JW, Feng T, Mørkedal B, Strand LB, Horn J, Mukamal K, et al. Obesity and risk for first ischemic stroke depends on metabolic syndrome: the HUNT study. Stroke. 2021;52(11):3555–61.
Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;378(9801):1485–92.
Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26:871–895, vii.
Howard VJ. Reasons underlying racial differences in stroke incidence and mortality. Stroke. 2013;44:S126-128.
Hunt KA, Gaba A, Lavizzo-Mourey R. Racial and ethnic disparities and perceptions of health care: does health plan type matter? Health Serv Res. 2005;40(2):551–76.
Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Piña IL, et al. Guidelines for the Prevention of Stroke in Women. Stroke. 2014;45:1545–1588.
Pourasgari M, Mohamadkhani A. Heritability for stroke: essential for taking family history. Casp J Intern Med. 2020;11(3):237–43.
Rundek T, Sacco RL. Risk factor management to prevent first stroke. Neurol Clin. 2008;26(4):1007–ix.
Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215–25.
Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993–1003.
SPS3 Investigators, Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367(9):817–25.
Sacco RL, Diener HC, Yusuf S, Cotton D, Ôunpuu S, Lawton WA, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359(12):1238–51.
Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331–7.
Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy–I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308(6921):81–106.
Sandercock PAG, Counsell C, Tseng MC, Cecconi E. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;3:CD000029.
The ESPS Group. The European stroke prevention study (ESPS). Principal end-points. Lancet. 1987;2(8572):1351–4.
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348(9038):1329–39.
Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375(1):35–43.
Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, et al. Cilostazol stroke prevention study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2000;9(4):147–57.
Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9(10):959–68.
McHutchison C, Blair GW, Appleton JP, Chappell FM, Doubal F, Bath PM, et al. Cilostazol for secondary prevention of stroke and cognitive decline: systematic review and meta-analysis. Stroke. 2020;51(8):2374–85.
Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. New England Journal of Medicine. 2017;377:1319–1330.
Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191–201.
Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380(20):1906–17.
Eikelboom JW, O’Donnell M, Yusuf S, Diaz R, Flaker G, Hart R, et al. Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J. 2010;159(3):348-353.e1.
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17.
Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84(2):527–39.
Pan KL, Singer DE, Ovbiagele B, Wu YL, Ahmed MA, Lee M. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6(7):e005835.
Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–9.
Pöss J, Desch S, Eitel C, de Waha S, Thiele H, Eitel I. Left ventricular thrombus formation after ST-segment-elevation myocardial infarction: insights from a cardiac magnetic resonance multicenter study. Circ Cardiovasc Imaging. 2015;8(10):e003417.
Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–14.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Stanifer JW, Pokorney SD, Chertow GM, Hohnloser SH, Wojdyla DM, Garonzik S, et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141(17):1384–92.
Shoamanesh A, Mundl H, Smith EE, Masjuan J, Milanov I, Hirano T, et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet [Internet]. 2022 [cited 2022 Sep 7];0(0). Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)01588-4/fulltext .
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104.
Peacock E, Krousel-Wood M. Adherence to antihypertensive therapy. Med Clin North Am. 2017;101(1):229–45.
Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–67.
Katsanos AH, Filippatou A, Manios E, Deftereos S, Parissis J, Frogoudaki A, et al. Blood pressure reduction and secondary stroke prevention: a systematic review and metaregression analysis of randomized clinical trials. Hypertension. 2017;69(1):171–9.
Klungel OH, Kaplan RC, Heckbert SR, Smith NL, Lemaitre RN, Longstreth WT, et al. Control of blood pressure and risk of stroke among pharmacologically treated hypertensive patients. Stroke. 2000;31(2):420–4.
Boan AD, Lackland DT, Ovbiagele B. Lowering of blood pressure for recurrent stroke prevention. Stroke. 2014;45(8):2506–13.
Bath PM, Woodhouse LJ, Appleton JP, Beridze M, Christensen H, Dineen RA, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391(10123):850–9.
SPS3 Study Group, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–15.
Mant J, McManus RJ, Roalfe A, Fletcher K, Taylor CJ, Martin U, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack: PAST-BP (Prevention After Stroke-Blood Pressure) randomised controlled trial. BMJ. 2016;24(352):i708.
SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16.
Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359(12):1225–37.
Eriksson S, Olofsson BO, Wester PO. Atenolol in secondary prevention after stroke. Cerebrovasc Dis. 1995;5(1):21–5.
PATS Collaborating Group. Post-stroke antihypertensive treatment study. A preliminary result. Chin Med J (Engl). 1995;108(9):710–7.
The Dutch TIA Trial Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke. Stroke. 1993;24(4):543–8.
Wang WT, You LK, Chiang CE, Sung SH, Chuang SY, Cheng HM, et al. Comparative effectiveness of blood pressure-lowering drugs in patients who have already suffered from stroke: traditional and Bayesian network meta-analysis of randomized trials. Medicine (Baltimore). 2016;95(15):e3302.
Chen GJ, Yang MS. The effects of calcium channel blockers in the prevention of stroke in adults with hypertension: a meta-analysis of data from 273,543 participants in 31 randomized controlled trials. PLoS ONE. 2013;8(3):e57854.
Wright JM, Musini VM, Gill R. First-line drugs for hypertension. Cochrane Database Syst Rev. 2018;2018(4):CD001841.
Chen R, Suchard MA, Krumholz HM, Schuemie MJ, Shea S, Duke J, et al. Comparative first-line effectiveness and safety of ACE (angiotensin-converting enzyme) inhibitors and angiotensin receptor blockers: a multinational cohort study. Hypertension. 2021;78(3):591–603.
Collins R, Armitage J, Parish S, Sleight P, Peto R, Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363(9411):757–67.
Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, et al. The Japan statin treatment against recurrent stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine. 2015;2(9):1071–8.
High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–59.
Tramacere I, Boncoraglio GB, Banzi R, Del Giovane C, Kwag KH, Squizzato A, et al. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: a systematic review and network meta-analysis. BMC Med. 2019;17(1):67.
Amarenco P, Benavente O, Goldstein LB, Callahan A, Sillesen H, Hennerici MG, et al. Results of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial by stroke subtypes. Stroke. 2009;40(4):1405–9.
Vavlukis M, Vavlukis A. Adding ezetimibe to statin therapy: latest evidence and clinical implications. Drugs Context. 2018;9(7):212534.
Giugliano RP, Pedersen TR, Saver JL, Sever PS, Keech AC, Bohula EA, et al. Stroke prevention with the PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor evolocumab added to statin in high-risk patients with stable atherosclerosis. Stroke. 2020;51(5):1546–54.
Jukema JW, Zijlstra LE, Bhatt DL, Bittner VA, Diaz R, Drexel H, Goodman SG, Kim Y-U, Pordy R, Reiner Ž, et al. Effect of Alirocumab on Stroke in ODYSSEY OUTCOMES. Circulation. 2019;140:2054–2062
Liu J, Wang LN. Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in people with stroke or transient ischaemic attack. Cochrane Database Syst Rev. 2019;10:CD010693.
Marsico F, Paolillo S, Gargiulo P, Bruzzese D, Dell’Aversana S, Esposito I, et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2020;41(35):3346–58.
Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet Lond Engl. 2003;361(9352):107–16.
North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–53.
Tan CH, Wu AG, Sia C-H, Leow AS, Chan BP, Sharma VK, Yeo LL, Tan BY. Cilostazol for secondary stroke prevention: systematic review and meta-analysis. Stroke Vasc Neurol. 2021;6:410–423.
de Havenon A, Sheth KN, Madsen TE, Johnston KC, Turan TN, Toyoda K, et al. Cilostazol for secondary stroke prevention: history, evidence, limitations, and possibilities. Stroke. 2021;52(10):e635-45.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
Dr. de Havenon reports NIH/NINDS funding (K23NS105924).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. de Havenon has received investigator initiated clinical research funding from the AAN, has received consultant fees from Integra and Novo Nordisk, royalty fees from UpToDate, and has equity in TitinKM and Certus.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bangad, A., Abbasi, M. & de Havenon, A. Secondary Ischemic Stroke Prevention. Neurotherapeutics 20, 721–731 (2023). https://doi.org/10.1007/s13311-023-01352-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-023-01352-w