Abstract
Introduction
Many clinical studies have proved the effectiveness of probiotics in metabolic disorders associated with insulin resistance. However, the impact of probiotic therapy on pancreatic β-cell function is ambiguous. The influence of probiotic supplementation vs. placebo on β-cell function in people with type 2 diabetes (T2D) was assessed in a double-blind, single-center, randomized, placebo-controlled trial (RCT).
Methods
Sixty-eight patients with T2D were selected for participation in the RCT. Patients were randomly allocated to consumption of live multistrain probiotics or a placebo for 8 weeks, administered as a sachet formulation in double-blind treatment. The primary main outcome was the assessment of β-cell function as change in C-peptide and HOMA-β (homeostasis model assessment-estimated β-cell function), which was calculated using the HOMA2 calculator (Diabetes Trials Unit, University of Oxford). Secondary outcomes were the changes in glycemic control-related parameters, anthropomorphic variables, and cytokines levels. Analysis of covariance was used to assess the difference between groups.
Results
Supplementation with live multiprobiotic was associated with slight significant improvement of β-cell function (HOMA-β increased from 32.48 ± 13.12 to 45.71 ± 25.18; p = 0.003) and reduction of fasting glucose level (13.03 ± 3.46 vs 10.66 ± 2.63 mmol/L and 234.63 ± 62.36 vs 192.07 ± 47.46 mg/dL; p < 0.001) and HbA1c (8.86 ± 1.28 vs 8.48 ± 1.22; p = 0.043) as compared to placebo. Probiotic therapy significantly affects chronic systemic inflammation in people with T2D by reducing pro-inflammatory cytokine levels.
Conclusions
Probiotic therapies modestly improved β-cell function in patients with T2D. Modulating the gut microbiota represents a new diabetes treatment and should be tested in more extensive studies.
Trial Registration
NCT05765292.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
The current understanding of the beneficial effects of probiotics in type 2 diabetes strictly relies on data which mainly focus on their impact on insulin resistance, glycemic control, and markers of chronic systemic inflammation. |
This double-blind, single-center, randomized, placebo-controlled trial was carried out to test the hypothesis if probiotic as compared to placebo could improve β-cell function in people with type 2 diabetes. |
What was learned from the study? |
Supplementation with live multiprobiotic as compared to placebo was associated with significant improvement of β-cell function and fasting glycemia. |
Introduction
Nowadays, the world is facing drastic changes with important consequences in the field of health, namely diseases of civilization: metabolic disorders, cardiovascular and endocrine diseases, age-related diseases, and cancer [1,2,3]. Each year the problem of obesity is becoming increasingly visible and discussed by some researchers [4]. The leading causes of obesity are an increased intake of energy-dense foods, excess sugar, flavor enhancers and trans fats in food, and reduced physical activity of the world’s population [5]. The consequences of obesity and obesity-related diseases, which are cardiovascular diseases, type 2 diabetes (T2D) and its complications, are among the first in the list of diseases causing death according to the World Health Organization [6,7,8].
T2D is a chronic disease in which the metabolism of carbohydrates, fats, and proteins is altered according to the inability of β-cells to secrete insulin and/or the peripheral cells failing to give a normal response to insulin [9]. This complicated process may involve genetic susceptibility and environmental and/or lifestyle factors. It will profoundly impact the demand for health care, economic costs, and quality of life [10]. Around 90% of people with diabetes worldwide have T2D. T2D is generally characterized by insulin resistance (IR), defined as the reduced ability of insulin to exert its biological effects on the target tissue and/or insufficient insulin production from pancreatic β-cells [11].
The relationship between alterations in intestinal microbiota and diseases of civilization is well known [12]. The gut microbiota is gaining meaningful scientific interest concerning obesity and different associated metabolic disorders in an effort to understand obesity’s etiology better and finds modern methods for its prevention and/or treatment [13,14,15,16].
Scientists studying the microbiome have discovered its new functions, mechanisms of regulation of metabolic processes, signaling pathways, and effector molecules that take part in them. The most studied and useful probiotics are lactic acid bacteria, especially bacteria from the genera Lactobacillus, Lactococcus, Streptococcus, and Enterococcus. Also, other bacterial species such as Bifidobacterium sp., Bacillus sp., Propionibacterium sp., Escherichia coli, and Saccharomyces have shown clinical efficiency [17,18,19]. The current understanding of the beneficial effects of probiotics in T2D strictly relies on animal and clinical data, which mainly focus on their impact on IR, anthropometric parameters, glycemic control, and markers of chronic systemic inflammation [11]. However, there is a lack of evidence-based probiotic efficacy on pancreatic β-cell function in terms of T2D and related metabolic disorders.
The rationale for probiotic choice was previously reported by our group in a series of comparative experimental investigations on the efficacy of mono- and multiprobiotic strains in metabolic disorders. We established the significantly lower prevalence of obesity [20] and more pronounced decrease in HOMA-IR, hepatic fat content, and proinflammatory cytokines levels for a live probiotic mixture as compared to lyophilized monoprobiotic strains [21, 22].
The current study assessed the influence of probiotic supplementation vs placebo on β-cell function in people with T2D in a double-blind, single-center, randomized, placebo-controlled trial (RCT). Therefore, the study will focus on the efficacy of live multistrain probiotics for protecting pancreatic β-cell damage in people with T2D.
Methods
This RCT was conducted at the Kyiv City Clinical Endocrinology Centre, Ukraine, between March 1 and December 15, 2021. The research protocol was approved by the local ethics committee (protocol 2/21) and put into practice on the basis of the Declaration of Helsinki (1975). The research was registered in the Clinical.Trial.gov database (NCT05765292). Before the RCT was initiated, its purpose and methods were clearly discussed with participants; thereafter, that all the patients voluntarily signed the informed consent.
Inclusion Criteria
The inclusion criteria were adult participants (aged 18–75 years) with proven T2D diagnosis based on the criteria of the American Diabetes Association (plasma glucose in fasting state ≥ 7.0 mmol/L; plasma glucose on random measurement ≥ 11.1 mmol/L; HbA1c ≥ 6.5% or glucose > 11.1 mmol/L 2 h after tolerance test with 75 g of glucose) [23]; presence of pancreatic β-cell dysfunction which was defined as HOMA2-β < 50% and treatment with insulin therapy alone or in combination with oral antidiabetic drugs (metformin and/or sulfonylureas) in a stable dose for at least 3 months prior to randomization; HbA1c level 6.5–11.0%; a signed informed consent.
Exclusion Criteria
The main exclusion criteria were presence of T1D; intake of antidiabetic drugs except for those specified in the inclusion criteria (pioglitazone, glucagon-like peptide 1 (GLP-1) analogues, dipeptidyl peptidase 4 (DPP4) inhibitors, etc.); severe diabetes-related complications at screening (i.e., end-stage diabetic kidney disease, neuropathy requiring pharmacological treatment, proliferative retinopathy, autonomic neuropathy); regular intake of probiotics, prebiotics, or antibiotics for 3 months prior to inclusion; previously diagnosed allergy to probiotics; gastrointestinal disorders including food allergy, gluten-sensitive enteropathy, ulcerative colitis; an uncontrolled cardiovascular or respiratory disease, an active malignant tumor or chronic infections; participant who had severe course of COVID-19 (extracorporeal membrane oxygenation, mechanically ventilated) and/or had a confirmed case of COVID-19 within 4 weeks prior to enrollment; participation in another clinical trial; pregnancy or lactation.
Study Design
Patients included with T2D included in the RCT were randomly allocated in a ratio of 1:1 to receive either probiotic or a placebo for 8 weeks. Randomization was double blind and carried out by an expert in statistics with blocks of four using a computer-generated list at www.randomization.com. The groups were homogeneous in terms of age, gender, and diagnosis. The co-investigators distributed the sachets among the participants according to their groups. The group allocation was blind both for the participants and the researchers. In addition, with a view to supporting the double-blind design, the statistics expert did not know the distribution of the participants between the study groups. The code was unblinded after the analysis had been completed and the database had been closed.
The preliminary randomization period was developed to reduce the effects of diet changes upon metabolic markers. For this purpose, 2 weeks before randomization all patients were offered to have a one-time session with a dietician to modify their lifestyle. The nutrition program included a corrective diet and drinking regime (daily norm 30–40 mL/kg). Patients were provided with a list of recommended and prohibited products. Any cooking method was recommended except for frying. The last meal was 1.5–2 h before bedtime. In addition, the participants were offered to continue their usual intake of antidiabetic drugs and to undertake standard average physical exercise for at least 1 h a day.
During the study 2-week follow-up, phone consultations were used to assess compliance, observance of the protocol requirements, and adverse event (AE) monitoring. In the event of a minor AE, patients were given the option to either continue or discontinue sachets but were nonetheless asked to complete follow-up visits. Project participants who had diarrhea, nausea/vomiting, sepsis, or took antibiotics during the research were not invited to the last visit.
Patient compliance was assessed by counting the remaining sachets and poll by the investigator at the end of treatment. The patient was considered a good match if less than 15% of the sachets remained during the count. The subject data were excluded from the final results if a participant had missed more than 15% of the suggested doses.
The rationale for study duration was based on our previous clinical studies in which the effectiveness of the same multiprobiotic for 8 weeks was proven [11, 24].
Drugs
The sachets containing probiotics or placebo had similar organoleptic characteristics, appearance, texture, weight, and smell. The only difference was on the specified number code printed on them. Multistrain probiotic Symbiter and the placebo were produced and delivered to the study center by N.D. Prolisok (Ukraine). The intervention contains of 14 live probiotic strains of Lactobacillus + Lactococcus (6 × 1010 CFU/g), Bifidobacterium (1 × 1010/g), Propionibacterium (3 × 1010/g), and Acetobacter (1 × 106/g) genera. Every patient after randomization received one pack (10 g) of Symbiter or placebo per day (QD) for an 8-week period. The developers of the drug delivered ready-packaged sachets to the hospital. Sachets were stored at a temperature of 4 ± 2 °C in a place inaccessible to children. The shelf life of the sachets was 2 months from the date of production. The probiotic company had guaranteed that the sachets contained live probiotic strains.
All the participants were instructed concerning the use of the supplementation, i.e., they were told to cut the pack as shown, then dissolve the contents in 15–30 mL of boiled drinking water of ambient temperature, stir thoroughly, and to consume it immediately after preparation.
Outcomes Assessment and Measurement
After informed consent was signed, the patients provided samples of their blood serum in a fasting state and these were immediately frozen at − 20 °C. Appropriate clinical and demographic information was collected for each person.
The primary outcome was the assessment of β-cell function as change in C-peptide and HOMA-β (%B, homeostasis model assessment-estimated β-cell function), which was calculated using the HOMA2 calculator. This model can be calculated using the software provided by the Oxford Centre for Diabetes, Endocrinology and Metabolism and available at http://www.dtu.ox.ac.uk/homacalculator/index.php. In addition, insulin sensitivity (%S) and HOMA2 index were secondary outcomes. C-peptide was measured using chemiluminescence immune analysis with the help of commercially available kits (Immulite, Siemens AG, Germany) with nanogram per milliliter scale.
The secondary outcomes of the RCT that were considered for investigating the efficiency of the probiotic therapy were glycemic control-related parameters, anthropometric variables, and markers of a chronic systemic inflammatory response, namely tumor necrosis factor (TNFα), interleukin (IL)-1β, IL-6, IL-8, and interferon-γ (INFγ). All parameters were determined by the hospital clinical laboratory after patients fasted for a 12-h period.
The glucose level in fasting state was determined by enzymatic Trinder method using an Exan device. HbA1c was measured using immunoturbidimetric analysis on a Cobas 6000 (Roche Diagnostics, Basel, Switzerland) with a reference range of 4.8–5.9%. HbA1c level was standardized with a reference method in keeping with DCCT (Diabetes Control and Complications Trial) and NGSP (National Glycohemoglobin Standardization Program) guidelines.
The level of proinflammatory cytokines (TNFα, IL-1β, IL-6, IL-8, INFγ) was determined using enzyme immunoassay with commercially available Sigma systems. Blood samples (5 mL) were taken in fasting state. Serum was stored at − 20 °C. Cytokine levels under consideration were measured in each sample and expressed in picograms per milliliter.
All the patients underwent anthropometry with the following data accumulated: body height (BH) accurate to 0.001 m; body weight (BW) accurate to 0.001 kg using medical scales. Body mass index (BMI) was calculated by Quetelet formula:
Waist circumference (WC) was measured using a flexible tape at the belly button level accurate to 0.001 m.
Similar methodologies have been presented before in recent RCTs [11, 24].
Statistical Analysis
Statistical analysis was done using SPSS version 20.0 (SPSS, Inc., Chicago, Illinois) and GraphPad Prism, version 6.0 (GraphPad Sofware, Inc., La Jolla, CA, USA). Quantitative changes were presented as the mean and standard deviation (SD). Qualitative changes were presented as percentages. The Kolmogorov–Smirnov one-sample test was used to test for normal distribution. Chi-squared was used to estimate the difference of the generated quantitative data. Changes in the participants’ outcomes after the initiation of therapy and end of the trial were compared by paired sample t tests (intragroup). Analysis of covariance (ANCOVA) was used to identify any differences between the two groups after intervention, adjusting for baseline measurements and confounders (BMI and sex) (intergroup). For primary endpoints we calculated effect size (ES) as post-intervention mean minus pre-intervention mean divided by pooled standard deviation. The value of ES was interpreted using Cohen’s d as follows: 0.2–0.5, small; 0.5–0.8, medium; and > 0.8, large. The efficacy analysis was performed on patients who completed the trial schedule, i.e., per-protocol (PP) population (n = 80). Approximately 85% of these patients formed the intention-to-treat (ITT) population which was used to perform analysis as a result of the loss of 12 patients because of non-compliance, COVID-19 pandemic, or other criteria.
Results
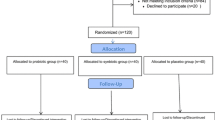
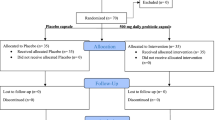
Patients were recruited into from March 1 until July 15, 2021, at the Kyiv City Clinical Endocrinology Center and Endocrinology Department of Bogomolets National Medical University. A total of 140 with T2D were enrolled from local electronic databases for conversation and assessment of compliance with the protocol criteria. Thirty-two patients were ineligible because of lack of protocol-specified criteria. The main reasons for exclusion were HOMA-β more than 50%, active COVID-19, and previous use of antibiotics and novel antidiabetic drugs. Twenty-eight patients refused to sign the informed consent after we explained the study’s purpose, methods, and design. Thus, 80 people with T2D and pancreatic β-cell dysfunction were included in the study. Participants were randomized into two groups: placebo (n = 40) and probiotic (n = 40). Overall, ithe ITT analysis included 68 people with T2D (n = 34, in each group). The main reasons for per-protocol exclusion are specified in the CONSORT flow diagram in Fig. 1.
All participants received standard care that included medical counseling, education in T2D, and lifestyle advice. Sixty-eight patients received more than 90% of the prescribed sachets in the double-blind treatment. One patient from the probiotic group was excluded from the study because of non-compliance (consumed only 62% of sachets). The patients were satisfied with the organoleptic features; both additives were tolerated well.
The groups were representative regarding age, gender, and T2D duration (Table 1). The enrolled patients’ baseline demographic and clinical characteristics did not significantly differ between groups (Table 1).
Project participants received standard therapy comprising oral antidiabetic drugs, insulin, or their combinations. As antidiabetic drugs such as metformin, peroxisome proliferator-activated receptor gamma (PPARγ) agonists, α-glucosidase inhibitors, GLP-1 agonists, DPP4 inhibitors, and sodium/glucose cotransporter (SGLT) inhibitors can change the gut microbiota composition [25, 26], before approving the research project we considered therapy with these antidiabetic drugs at least 3 months before randomization as an exclusion criterion. However, today, metformin is acknowledged as the first-line therapy in T2D, so it would be unethical to exclude it from the participants’ treatment. For our study, we randomly selected patients who had been on a stable dose of metformin for at least 4 weeks before starting the study. We found no significant difference in the daily average dose between the two groups (as shown in Table 1). Both groups were similar regarding the parameters that were used to measure primary and secondary outcomes (Table 2).
Primary Outcomes Analysis
The primary outcome was an improvement of β-cell function as a change %B from baseline to 8-week follow-up after probiotic therapy in intragroup analysis (Table 2, Fig. 2a). There was a significant increase of %B (32.48 ± 13.12 vs 45.71 ± 25.18; p = 0.003) after 8 weeks of treatment period. In the placebo group, the intervention was associated with non-significant improvement of %B (34.51 ± 12.07 vs 38.96 ± 18.07; p = 0.122). Moreover, we observed medium ES for the probiotic group (Cohen’s d = 0.65) and small for placebo (Cohen’s d = 0.28). The ES on C-peptide values in both groups was less than 0.2. On the other hand, ANCOVA did not show significant differences between the mean changes of the primary endpoint (− 4.45 ± 16.32 vs − 13.28 ± 24.48; p = 0.085). Also, in both groups, no statistically significant changes were found in the other primary outcome parameter, namely C-peptide level (Table 2, Fig. 2b).
Secondary Outcomes Analysis
Other indicators also confirm the effectiveness of probiotic therapy. There were significant reductions of fasting glucose level (13.03 ± 3.46 vs 10.66 ± 2.63 mmol/L and 234.63 ± 62.36 vs 192.07 ± 47.46 mg/dL; p < 0.001) and HbA1c (8.86 ± 1.28 vs 8.48 ± 1.22; p = 0.043) after 8 weeks of the treatment period by probiotic (Fig. 3a–c). Improvement in glycemia was also confirmed in the mean changes of groups by ANCOVA (0.28 ± 2.41 vs 2.36 ± 3.57 mmol/L and 5.08 ± 43.46 vs 42.56 ± 64.29 mg/dL; p = 0.006). In the placebo group, these parameters change insignificantly after treatment. In both groups, the intervention did not significantly influence insulin sensitivity (Table 2, Fig. 4a, b).
In the context of the secondary outcomes analysis, changes in anthropometric indices were investigated. The main anthropometric parameters assessed showed a significant reduction of WC in the probiotic group from 98.32 ± 10.47 to 97.92 ± 9.99 (p = 0.039) (Table 3, Fig. 5c). BW and BMI within both intervention groups changed insignificantly (Fig. 5a, b). In the between-group analysis, the mean changes from the baseline were compared, and all anthropometric variables changed insignificantly (Table 3).
Probiotic therapy had a significant effect on the chronic systemic inflammation in people with T2D. In the probiotic group, there were statistically significantly decreases in IL-1β (35.34 ± 19.60 vs 30.15 ± 14.79; p = 0.008), TNFα (44.41 ± 18.21 vs 38.73 ± 18.28; p = 0.001), and INFγ (191.80 ± 65.74 vs 177.56 ± 53.46; p = 0.028) (Table 4, Fig. 6). The concentration of IL-8 statistically did not change in both groups after intervention. On the other hand, the IL-6 level significantly decreased in both probiotic (20.69 ± 11.88 vs 16.88 ± 10.10; p = 0.017) and placebo (17.90 ± 11.64 vs 15.07 ± 9.52; p = 0.048) groups after 8 weeks of treatment (Table 4, Fig. 7). It was accompanied by a significant reduction of proinflammatory cytokines within intergroup comparison during the treatment, namely IL-1β (p = 0.045) and TNFα (p = 0.003) correspondingly (Table 4).
Adverse Events
Five patients discontinued the study permanently as a result of COVID-19-associated pneumonia which required hospitalization and oxygen therapy. These AEs were stated as serious, but not related to intervention. The main reported AEs were gastrointestinal complaints, which were transient and mild in their intensity. In the probiotic group, five patient experienced AE (short-term increase in abdominal pain and bloating, n = 2; diarrhea, n = 2; and constipation, n = 1). AEs reported by patients receiving placebo were nausea (n = 1), heartburn (n = 2), and transient diarrhea (n = 2). The overall incidence of AEs, including COVID-19 complications, was the same in both groups and constituted 20.5%.
Discussion
Modern studies of the relationship between the human microbiome and the state of health of the host make it possible to develop new methods of treatment for various diseases. Dysbiosis relates to increased gut permeability, which results in elevated levels of bacterial endotoxins such as lipopolysaccharides (LPS) and thus increasing inflammation. Chronic inflammation leads to different metabolic disease: TD2, metabolic syndrome, obesity, and insulin resistance [27,28,29,30,31]. Today, a lot of data has appeared indicating a direct relationship between gut microbiota changes and the development of T2D [32,33,34]. However, evidence-based probiotic efficacy on pancreatic β-cell function in people with T2D is lacking.
The inability of β-cells to produce enough insulin in response to glycemic load leads to T2D. Pancreatic cells lose their activity as a result of a decrease in the number of cells themselves and a decrease in their function [35]. It is well known that the various pathways act synergistically to result in β-cell dysfunction [36]. Therefore, by regulating the microbiome, it is possible to improve the carbohydrate metabolism of the host [37]. This can be done through the use of probiotics [38]. Moreover, antibiotic therapy is directly connected with a higher risk of several diseases, including T2D [39, 40]. In vivo and in vitro diet-induced T2D models were used to study the molecular effects of the gut microbial metabolite trimethylamine N-oxide (TMAO) on functional β-cell mass. TMAO normalized glucolipotoxicity-mediated damage in β-cells and primary islet function [41]. It was found that gut microbial metabolites of dietary flavonoids (hippuric acid, HA; homovanillic acid, HVA; and 5-phenylvaleric acid, 5PVA) exert potent protective activities in β-cells [42]. All metabolites increased glucose-stimulated insulin secretion (GSIS) but did not influence β-cell mitochondrial respiration or components of the mitochondrial electron transport chain [42]. A study in olanzapine-induced diabetic mice reported improved insulin sensitivity and glucose tolerance by downregulating phosphoenolpyruvate carboxykinase (PEPCK) and G6PC [43]. Experiments also showed that multistrain probiotic (Probioglu™) administration increased the β-cell mass and decreased inflammation and oxidative stress [44]. However, the researchers point to the need for clinical trials to confirm the established effects of the probiotic supplement.
Before in our clinical investigation, it was found that people with obesity and T2D who used a multistrain probiotic for 8 weeks experienced significant reductions in HOMA-IR, BW, BMI, waist circumference, and cytokine content (TNFα, IL-1β, IL-6). However, only reductions in waist circumference and serum levels of TNFα and IL-1β were confirmed in an ANCOVA intergroup analysis. The glycemic control parameters did not show statistically significant differences in intra- or intergroup analysis. A sub-analysis was conducted, focusing on changes in HbA1c based on the patient’s response to treatment in each group. A positive effect was observed in patients with decreased HOMA-IR, and a significant reduction in HbA1c was found only in respondents from the probiotic therapy group [45]. This RCT assessed the efficacy and safety of a live multistrain probiotic in managing T2D by estimation of β-cell function. Supplementation with live multiprobiotic was associated with significant improvement of β-cell function and fasting glycemia. Prebiotic metabolites exert potent protective activities in β-cells and enhanced glucose-stimulated insulin secretion [42, 46]. Short-chain fatty acids (SCFAs), in the main acetate, butyrate and propionate, are synthesized through bacterial fermentative processes of dietary carbohydrates by such strains as Bacteroidales S24-7, Parabacteroides, Mucispirillum, and Coprococcus [47]. SCFAs are signaling and energy molecules in the bowel and peripheral organs. They regulate energy and glucose homeostasis [48, 49]. Butyrate improves insulin sensitivity and acetate modulates secretion of insulin from pancreatic β-cells [50]. Extensive studies have been carried out to understand the mechanisms responsible for initiating the functionalities of these SCFAs toward body tissues, which greatly involves the SCFA-specific receptors free fatty acid receptor 2 (FFAR2) and free fatty acid receptor 3 (FFAR3). SCFA signaling by FFA2 and FFA3 is known now to affect not only insulin secretion but also β-cell survival and proliferation and represents an exciting novel link between the gut microbiota and the β-cells [51, 52].
For several decades, it has been believed that increased inflammatory tone greatly affects glucose metabolism. Chronic inflammation is accompanied by oxidative stress which results in β-cell dysfunction and α-cell expansion in the pancreas, which lead to the progression of T2D in obese subjects, and gut microbiota influences the secretion of proinflammatory cytokines [53,54,55]. It is known that gut bacteria can indirectly stimulate proinflammatory cytokine production by the host through their metabolites or reduce inflammation by synthesizing anti-inflammatory substances [56]. Indeed, the present study found that inflammatory biomarkers (IL-1β, TNFα, IL-6, INFγ) improved after using a live multistrain probiotic supplement compared to the placebo.
To our knowledge, the current study is the first to assess the effect of probiotic supplement on pancreatic β-cell function as a primary endpoint in T2D. Previously, several preclinical studies reported that immunomodulatory probiotic effects may be beneficial for β-cell survival and regeneration in T1D animal models [57, 58]. Administration of IRT5 (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus reuteri, Bifidobacterium bifidium, Streptococcus thermophiles) to non-obese diabetic (NOD) mice, a classical animal model of human T1D, reduced insulitis score, but conversely increased β-cell mass and led to significantly lower incidence of diabetes [57]. Consuming IRT5 decreases intestinal permeability substantially. Additionally, it increased the percentage of gut-homing receptor-expressing CCR9+ regulatory T (Treg) cells in both the pancreatic lymph nodes (PLNs) and the lamina propria of the small intestine (SI-LP) [57]. This study offers a new understanding of how IL-10 affects neutrophils and CD4+ T cells in BDC2.5+ NOD mice and demonstrates the significant connection between gut microbiota and neutrophils in the development of T1D [58]. Bedi et al. discovered significant similarities between human and bacterial glutamic acid decarboxylase (GAD). As a result, these immune cells may attack human β-cells, ultimately causing the development of T1D [59]. Conversely, it was found that Lactobacillus rhamnosus GG and Bifidobacterium lactis BB-12 did not significantly preserve the remaining pancreatic β-cell function in children recently diagnosed with T1D [60].
Probiotic therapy is considered a promising approach to manage metabolic disorders. It works by restoring the composition and health of the microbiota and impacting specific mechanisms to maintain overall health. This therapy is safe, with minimal side effects, and can be used for an extended period. It may help regulate glucose metabolism, enhance β-cell function, and reduce chronic inflammation in individuals with T2D.
The main limitation of this study was the unidentified composition of the intestinal microbiota in view of defining the personalized impact upon the changes of metabolic parameters. Furthermore, we did not estimate any changes of the levels SCFAs: this could help find out the links between the use of probiotics and pancreatic β-cell function improvement. The latter should be taken into consideration in future studies.
Conclusion
Probiotic therapies have a slight positive effect on the function of β-cells in individuals with T2D. This highlights the potential of modulating the gut microbiota as a new form of diabetes treatment, which should be further explored through extensive studies.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Falalyeyeva T, Komisarenko I, Yanchyshyn A, et al. Vitamin D in the prevention and treatment of type-2 diabetes and associated diseases: a critical view during COVID-19 time. Minerva Biotechnol Biomol Res. 2021;33:65–75.
Gruber H-J, Semeraro MD, Renner W, Herrmann M. Telomeres and age-related diseases. Biomedicines. 2021;9:1335.
Korotkyi OH, Luhovska TV, Serhiychuk TM, Dvorshchenko KO, Falalyeyeva TM, Ostapchenko LI. The gut microbiota of rats under experimental osteoarthritis and administration of chondroitin sulfate and probiotic. Mikrobiol Z. 2020;82:64–73.
Mykhalchyshyn G, Kobyliak N, Bodnar P. Diagnostic accuracy of acyl-ghrelin and it association with non-alcoholic fatty liver disease in type 2 diabetic patients. J Diabetes Metab Disord. 2015;14:44.
Tokarchuk A, Abenavoli L, Kobyliak N, et al. Nutrition program, physical activity and gut microbiota modulation: a randomized controlled trial to promote a healthy lifestyle in students with vitamin D3 deficiency. Minerva Med. 2022;113:683–94.
Ali MK, Pearson-Stuttard J, Selvin E, Gregg EW. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia. 2022;65:3–13.
Kelsey MD, Nelson AJ, Green JB, et al. Guidelines for cardiovascular risk reduction in patients with type 2 diabetes: JACC guideline comparison. J Am Coll Cardiol. 2022;79:1849–57.
Han H, Cao Y, Feng C, et al. Association of a healthy lifestyle with all-cause and cause-specific mortality among individuals with type 2 diabetes: a prospective study in UK biobank. Diabetes Care. 2022;45:319–29.
Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes Targets Ther. 2014;7:587–91.
Eslami M, Bahar A, Hemati M, et al. Dietary pattern, colonic microbiota and immunometabolism interaction: new frontiers for diabetes mellitus and related disorders. Diabet Med. 2020;38:e14415.
Kobyliak N, Abenavoli L, Falalyeyeva T, Kovalchuk O, Kyriienko D, Komisarenko I. Metabolic benefits of probiotic combination with absorbent smectite in type 2 diabetes patients a randomised controlled trial. Rev Recent Clin Trials. 2021;16:109–19.
Moszak M, Szulińska M, Bogdański P. You are what you eat—the relationship between diet, microbiota, and metabolic disorders—a review. Nutrients. 2020;12:1096.
Kobyliak N, Falalyeyeva T, Bodnar P, Beregova T. Probiotics supplemented with omega-3 fatty acids are more effective for hepatic steatosis reduction in an animal model of obesity. Probiot Antimicrob Proteins. 2017;9:123–30.
Hijová E. Synbiotic supplements in the prevention of obesity and obesity-related diseases. Metabolites. 2022;12:313.
Kobyliak N, Falalyeyeva T, Kyriachenko Y, et al. Akkermansia muciniphila as a novel powerful bacterial player in the treatment of metabolic disorders. Minerva Endocrinol. 2022;47:242–52.
Korotkyi O, Vovk A, Galenova T, et al. Effect of probiotic on serum cytokines and matrix metalloproteinases profiles during monoiodoacetate-induced osteoarthritis in rats. Minerva Biotecnol. 2019;31:68–73.
Hampe CS, Roth CL. Probiotic strains and mechanistic insights for the treatment of type 2 diabetes. Endocrine. 2017;58:207–27.
Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70:2782–858.
Kobyliak N, Falalyeyeva T, Beregova T, Spivak M. Probiotics for experimental obesity prevention: focus on strain dependence and viability of composition. Endokrynol Pol. 2017;68(6):659–67.
Kobyliak N, Falalyeyeva T, Virchenko O, et al. Comparative experimental investigation on the efficacy of mono- and multiprobiotic strains in non-alcoholic fatty liver disease prevention. BMC Gastroenterol. 2016;15(16):34.
Kobyliak N, Falalyeyeva T, Tsyryuk O, et al. New insights on strain-specific impacts of probiotics on insulin resistance: evidence from animal study. J Diabetes Metab Disord. 2020;19:289–96.
Voidarou C, Antoniadou M, Rozos G, et al. Fermentative foods: microbiology, biochemistry, potential human health benefits and public health issues. Foods. 2020;10:69.
Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care. 2019;42:S13–28.
Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, et al. Probiotic and omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance, improves glycemia and obesity parameters in individuals with type 2 diabetes: a randomised controlled trial. Obes Med. 2020;19:100248.
Kyriachenko Y, Falalyeyeva T, Korotkyi O, Molochek N, Kobyliak N. Crosstalk between gut microbiota and antidiabetic drug action. World J Diabetes. 2019;10:154–68.
Montandon SA, Jornayvaz FR. Effects of antidiabetic drugs on gut microbiota composition. Genes. 2017;8:250.
Salari A, Mahdavi-Roshan M, Kheirkhah J, Ghorbani Z. Probiotics supplementation and cardiometabolic risk factors: a new insight into recent advances, potential mechanisms, and clinical implications. Pharma Nutr. 2021;16: 100261.
Kesika P, Sivamaruthi BS, Chaiyasut C. Do probiotics improve the health status of individuals with diabetes mellitus? A review on outcomes of clinical trials. Biomed Res Int. 2019;2019:1531567.
Torres S, Fabersani E, Marquez A, Gauffin-Cano P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur J Nutr. 2019;58:27–43.
Wang G, Liu J, Xia Y, Ai L. Probiotics-based interventions for diabetes mellitus: a review. Food Biosci. 2021;43: 101172.
Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72.
Gurung M, Li Z, You H, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590.
Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71.
Bordalo Tonucci L, Dos Santos KMO, De Luces Fortes Ferreira CL, Ribeiro SMR, De Oliveira LL, Martino HSD. Gut microbiota and probiotics: focus on diabetes mellitus. Crit Rev Food Sci Nutr. 2017;57:2296–309.
Christensen AA, Gannon M. The beta cell in type 2 diabetes. Curr Diabetes Rep. 2019;19:81.
Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16:349–62.
Martin AM, Sun EW, Rogers GB, Keating DJ. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front Physiol. 2019;10:428.
Rad A. The future of diabetes management by healthy probiotic microorganisms. Curr Diabetes Rev. 2017;13:582–9.
Yuan J, Hu YJ, Zheng J, et al. Long-term use of antibiotics and risk of type 2 diabetes in women: a prospective cohort study. Int J Epidemiol. 2020;49:1572.
Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab. 2015;100:3633–40.
Krueger ES, Beales JL, Russon KB, et al. Gut metabolite trimethylamine N-oxide protects INS-1 β-cell and rat islet function under diabetic glucolipotoxic conditions. Biomolecules. 2021;11:1892.
Bitner BF, Ray JD, Kener KB, et al. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in β-cells and skeletal muscle cells. J Nutr Biochem. 2018;62:95–107.
Huang D, Gao J, Li C, et al. A potential probiotic bacterium for antipsychotic-induced metabolic syndrome: mechanisms underpinning how Akkermansia muciniphila subtype improves olanzapine-induced glucose homeostasis in mice. Psychopharmacology. 2021;238:2543–53.
Hsieh P-S, Ho H-H, Tsao SP, et al. Multi-strain probiotic supplement attenuates streptozotocin-induced type-2 diabetes by reducing inflammation and β-cell death in rats. PLoS ONE. 2021;16:e0251646.
Braga RM, Dourado MN, Araújo WL. Microbial interactions: ecology in a molecular perspective. Braz J Microbiol. 2016;47:86–98.
Li Q, Hu J, Nie Q, et al. Hypoglycemic mechanism of polysaccharide from Cyclocarya paliurus leaves in type 2 diabetic rats by gut microbiota and host metabolism alteration. Sci China Life Sci. 2021;64:117–32.
Wang G, Si Q, Yang S, et al. Lactic acid bacteria reduce diabetes symptoms in mice by alleviating gut microbiota dysbiosis and inflammation in different manners. Food Funct. 2020;11:5898–914.
Kreznar JH, Keller MP, Traeger LL, et al. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Rep. 2017;18:1739–50.
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45.
Priyadarshini M, Villa SR, Fuller M, et al. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol Endocrinol. 2015;29:1055–66.
Priyadarshini M, Navarro G, Layden BT. Gut microbiota: FFAR reaching effects on islets. Endocrinology. 2018;159:2495–505.
Rosli NSA, Abd Gani S, Khayat ME, Zaidan UH, Ismail A, Abdul Rahim MBH. Short-chain fatty acids: possible regulators of insulin secretion. Mol Cell Biochem. 2023;478:517–30.
Eguchi K, Nagai R. Islet inflammation in type 2 diabetes and physiology. J Clin Investig. 2017;127:14–23.
Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol. 2020;16:81–90.
Eguchi N, Vaziri ND, Dafoe DC, Ichii H. The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int J Mol Sci. 2021;22:1–18.
Hasain Z, Mokhtar NM, Kamaruddin NA, et al. Gut microbiota and gestational diabetes mellitus: a review of host-gut microbiota interactions and their therapeutic potential. Front Cell Infect Microbiol. 2020;10:188.
Kim TK, Lee JC, Im SH, Lee MS. Amelioration of autoimmune diabetes of NOD mice by immunomodulating probiotics. Front Immunol. 2020;11:1832.
Huang J, Tan Q, Tai N, et al. IL-10 deficiency accelerates type 1 diabetes development via modulation of innate and adaptive immune cells and gut microbiota in BDC2.5 NOD mice. Front Immunol. 2021;12:702955.
Bedi S, Richardson TM, Jia B, Saab H, Brinkman FSL, Westley M. Similarities between bacterial GAD and human GAD65: implications in gut mediated autoimmune type 1 diabetes. PLoS ONE. 2022;17:e0261103.
Groele L, Szajewska H, Szalecki M, et al. Lack of effect of Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12 on beta-cell function in children with newly diagnosed type 1 diabetes: a randomised controlled trial. BMJ Open Diabetes Res Care. 2021;9:e001523.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
MS and NK contributed to the conceptualization and the original idea of this manuscript. DK and TF contributed to methodology and reviewed the literature. MS, IK and TF were involved in validation and revised and validated the literature findings. MS performed formal analysis. NK and DK contributed to investigations. DK was involved in data curation. MS, TF and NK contributed to writing—original draft preparation. TF contributed to writing—review and editing. IK did the visualization. IK, OK and NK were involved in supervision. NK and MS contributed to project administration. All authors contributed to the article and approved the final manuscript version.
Corresponding author
Ethics declarations
Conflict of Interest
Maryana Savytska, Nazarii Kobyliak, Dmytro Kyriienko, Tetyana Falalyeyeva, Iuliia Komisarenko, and Oleksandr Kovalchuk declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Approval
The research protocol was approved by the local ethics committee (protocol 2/21) and put into practice based on the Declaration of Helsinki (1975). The present study was conducted in compliance with the principles of secrecy and confidentiality. The collection and analysis of the patients’ information were done anonymously and using a code to prevent disclosure. Patients were free to leave the study at any time during the study, without the need to provide any explanation or reason.
Additional information
Prior Presentation: The preliminary results of the current study were presented in a poster session at the 21st European Congress of Endocrinology (Lyon, France, 18–21 May, 2019). https://doi.org/10.1530/endoabs.63.P593.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Savytska, M., Kyriienko, D., Komisarenko, I. et al. Probiotic for Pancreatic β-Cell Function in Type 2 Diabetes: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Diabetes Ther 14, 1915–1931 (2023). https://doi.org/10.1007/s13300-023-01474-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01474-6