Abstract
Introduction
Preference for quality of life is important in deciding the treatment strategy for patients with type 2 diabetes mellitus. This study aimed to assess the effect of omarigliptin on patients’ psychological attitudes and responses compared with daily dipeptidyl peptidase-4 inhibitors (DPP4is) by measuring the burden of pharmacotherapy using the Diabetic Treatment Burden Questionnaire (DTBQ).
Methods
Patients with type 2 diabetes mellitus who were taking daily DPP-4is were enrolled and randomized to a group that switched to omarigliptin or a group that continued daily DPP4is and were monitored for 12 weeks. The primary endpoint was the change in the DTBQ score from baseline to week 12. The secondary endpoints included changes in blood test results, medication preferences and medication adherence.
Results
The DTBQ total score significantly decreased from baseline to week 12 in both groups; however, no significant intergroup differences were observed. The DTBQ subscale, implementation and flexibility burden scores significantly decreased in the group that switched to omarigliptin, although no significant intergroup difference in the change was observed. DTBQ scores and medication preferences were associated with improvements in the DTBQ scores.
Conclusion
Although this study failed to demonstrate the improvement of DTBQ total score by switching from daily DPP4is to omarigliptin compared with continuing the daily DPP4is, the DTBQ subscale score implementation and flexibility burden score were significantly improved only in the group that switched to omarigliptin, suggesting the possibility of switching from daily DPP4is to omarigliptin to decrease the patients’ medication burden.
Trial Registration
jRCTs031200437.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Patients' preference and quality of life are important in deciding the treatment strategy for patients with type 2 diabetes mellitus. |
This study aimed to assess the effect of omarigliptin on patients’ psychological attitudes and responses compared with daily dipeptidyl peptidase-4 inhibitors (DPP4is) by measuring the burden of pharmacotherapy using the Diabetic Treatment Burden Questionnaire (DTBQ). |
What was learned from the study? |
Although the change in the DTBQ total score did not differ between the groups that switched to omarigliptin and the group that continued daily DPP4is, the DTBQ subscale scores, implementation and flexibility burden scores significantly decreased in the group that switched to omarigliptin. |
The results in this study may suggest the possibility of switching from daily DPP4is to omarigliptin to decrease the patients’ medication burden. |
Introduction
Dipeptidyl peptidase-4 inhibitor (DPP4i) is a class of oral hypotensive agent (OHA) that carries a relatively low risk of hypoglycemia [1, 2]. Since DPP4is are more effective, especially in the East Asian population [3], they have become the most frequently prescribed OHA in Japan [4,5,6].
At the time of designing this study, physicians selected antidiabetic agents depending on the pathological condition of each patient, and the treatment guidelines for diabetes mellitus in Japan lack clear medication strategies [7, 8]. However, the current guidelines in Japan recommend pharmacotherapy considering appropriate targets for glucose control: pathological conditions such as obesity, safety, additional benefits regarding complications and other relevant factors, including medication adherence and medication costs, to prevent or suppress complications and maintain patients’ quality of life (QOL) [9]. The guidelines issued by the American Diabetes Association (ADA) also recommend focusing on the preference or QOL of patients in addition to the pathological conditions and efficacy, side effects and costs of medical agents [10]. The World Health Organization (WHO) warns of poor medication adherence in patients with a long duration of chronic illnesses [11]. For example, although metformin has the benefit of suppressing cardiovascular events [12] and was recommended as a first-line therapy for type 2 diabetes mellitus according to the consensus guidelines of the ADA and the European Association for the Study of Diabetes (EASD) at the planning of this study [13], a meta-analysis reported poor adherence to metformin compared with sulfonylurea [14]. Worsening blood glucose control and the onset of diabetic complications are factors that affect patient QOL [15, 16].
Omarigliptin is a once-weekly DPP4i launched in November 2015 in Japan. Omarigliptin has several characteristics: (1) it is minimally metabolized in the liver, (2) it is not deposited in a specific tissue and is distributed widely in the body, resulting in a low filtration rate in the kidney, and (3) when it is filtered in the renal glomeruli, approximately 60% of it is reabsorbed in the renal tubule in its unchanged form [17]. These characteristics result in stable inhibition of dipeptidyl peptidase-4 for a week after administration [18, 19] and persistent improvements in glycemic control with a safety profile compared to daily DPP4is [20]. Since dulaglutide, a once-weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA), improved patient QOL compared to placebo or twice-daily GLP-1 RA [21], omarigliptin, a once-weekly DPP4i, might also contribute to improving patient QOL. Therefore, this study aimed to assess the effect of omarigliptin on patients’ psychological attitudes and responses compared with conventional once- or twice-daily DPP4is by measuring the burden of pharmacotherapy using the Diabetic Treatment Burden Questionnaire (DTBQ) [22].
Methods
Study Design
This study of omarigliptin, weekly DPP-4i, to evaluate the effect on psychological attitudes and responses compared with daily DPP-4is in patients with type 2 diabetes mellitus (ONWARD-DPP4 study) was a multicenter, open-label, randomized controlled trial conducted at 24 medical institutions in Japan under management of the Japan Society for Patient-Reported Outcome (PRO). Patient enrollment was conducted between March 2021 and December 2021, and each enrolled patient was followed up for 12 weeks. This study, including its protocols and all participating medical institutions, was inspected and approved by the Japan Physicians Association Clinical Research Review Board, which is certified by the Minister of Health, Labor, and Welfare in Japan, according to the requirements of the Clinical Trials Act. This study was registered in the Japan Registry of Clinical Trials (registration no. jRCTs031200437) after receiving approval from a certified review board, according to the requirements of the Clinical Trials Act. The study was conducted in accordance with the Declaration of Helsinki, Clinical Trials Act and other current legal regulations in Japan. Written informed consent was obtained from all enrolled patients who met the eligibility criteria prior to treatment.
Patient Population
The inclusion criteria were as follows: (1) patients with type 2 diabetes mellitus who use once- or twice-daily DPP-4is; (2) those who did not change the anti-diabetic agents (dose, usage or type) within 8 weeks before giving their consent; (3) male or female patients aged 20 years or older; a(4) patients who provided written informed consent. The exclusion criteria were as follows: (1) patients who use any combination tablets with DPP-4i; (2) patients who have a history of using omarigliptin or trelagliptin; (3) patients who use ≥ 10 pharmaceutical agents; (4) patients whose HbA1c was ≥ 10% upon giving their consent; (5) patients who had a history of severe hypoglycemia within a year before giving their consent; (6) patients with type 1 diabetes mellitus or secondary diabetes; (7) patients who routinely use any infusions such as insulin or GLP-1 receptor agonist; (8) patients with cognitive dysfunction or psychiatric disease; (9) patients with alcoholism or drug addiction; (10) patients in the perioperative period or with serious infection or injury; (11) patients with atrial fibrillation or frequent premature ventricular contraction; (12) patients with moderate-to-severe heart failure [class 3 or worse based on the New York Heart Association (NYHA) Functional Classification]; (13) patients with moderate-to-severe renal function (male: serum creatinine > 1.4 mg/dl, female: serum creatinine > 1.2 mg/dl; (14) patients with ascitic fluid or severe hepatic function (AST ≥ 100 IU/l); (15) patients with a history of poorly controlled hypertension or dyslipidemia within 12 weeks before giving their consent; (16) patients with contraindicating conditions to using the study agents; (17) patients who are pregnant, breastfeeding, possibly pregnant or planning to be pregnant; (18) patients who need a legal representative for giving consent; (19) patients with other conditions that the responsible investigator or subinvestigators thought made it inappropriate to participate in the study.
Randomization and Study Intervention
To balance the number of daily DPP4i medications across the groups, equal numbers of patients who used once daily DPP-4is and those who used twice daily DPP-4is were enrolled in this study. After obtaining informed consent, eligible patients were randomly assigned to either group to switch to omarigliptin or the group to continue daily DPP4is at a ratio of approximately 1:1. Randomization was performed using a computer-based dynamic allocation method with a minimization procedure to balance the two allocation factors (HbA1c level and age) across the groups. Patients who were assigned to the group to switch to omarigliptin discontinued the daily DPP4is, which were used after they had given their consent and switched to omarigliptin. Patients who were assigned to the group to continue daily DPP4is continued to take daily DPP4is, which were used at giving their consent. The patients were followed up for 12 weeks, with observation points at baseline and week 12. The detailed observation schedules and items are listed in Supplementary Table 1.
Study Outcomes
The primary endpoint was the change in DTBQ score from baseline to week 12. DTBQ is a specific questionnaire to assess the burden of pharmacotherapy on patients with type 2 diabetes mellitus, composed of 18 questions and three subscales: implementation burden (sum of item scores 1–10), flexibility burden (sum of item scores 11–13) and blood glucose control burden (sum of item scores 14–18) [22]. Each item is scored on a 7-point Likert scale ranging from (1) strongly disagree, (2) mostly disagree, (3) slightly disagree, (4) neither agree nor disagree, (5) slightly agree, (6) mostly agree to (7) strongly agree. Since larger answer number means a heavier treatment burden in items 1–10, answer numbers were converted to 0–6; 0 indicates minimum and 6 maximum treatment burden. In contrast, because a larger answer number indicates a smaller treatment burden in items 11–18, the answer numbers were inverted and then converted to 0–6. Finally, the DTBQ total, implementation burden, flexibility burden and blood glucose control burden scores ranged from 0 to 108, 0 to 60, 0 to 18 and 0 to 30, respectively. The secondary endpoints included changes in blood tests, medication preferences, medication adherence and frequency of any adverse events. The DTBQ and medication preferences were answered by the study participants on a paper questionnaire. Medication adherence was measured using a paper medication diary recorded by the participants. Other clinical outcomes were collected from investigators’ case report forms.
Sample Size Calculation
In a previous study, the total score of DTBQ was 21.1 ± 12.9 in patients who were taking once-daily oral hypoglycemic agents (OHAs), 33.9 ± 15.8 for those taking OHAs twice daily or more and 17.0 ± 12.0 for those taking once weekly OHAs. Based on these previous results, we assumed that the total score of DTBQ at baseline, change in the DTBQ total score in the group that switched to omarigliptin, change in the group to continue daily DPP4is and the standard deviation in change in this study were 27.5, − 10.5, 0 and 21.0, respectively. Under these assumptions, 86 patients per group provided a power of 90% to detect intergroup differences using a two-sided t-test at 5% significance. The dropout rate was estimated to be approximately 20%. Thus, 108 participants were required per group, yielding a total sample size of 216 participants.
Statistical Analysis
Analyses of the primary and secondary endpoints were performed on the full analysis set (FAS), which included all patients assigned to the study intervention. However, patients with a significant violation of the study protocol (e.g., registration without consent or registration outside the enrollment period) were excluded from the FAS. Sensitivity analysis was performed using the per-protocol set by excluding patients with protocol violations, such as violation of eligibility criteria, use of prohibited drugs or poor medication adherence to the study or control agent (< 75% or > 120%). The safety analysis included all treated patients. All tests were two sided, and statistical significance was set at p < 0.05. The primary endpoint, the change in DTBQ score from baseline to week 12, was tested using analysis of covariance (ANCOVA), with the treatment groups as the fixed effect and allocation factors (HbA1c and age at registration) as covariates. Summary statistics for measurement and change from baseline were calculated for the analysis of secondary endpoints. The one-sample t-test or Wilcoxon signed-rank test for intragroup comparison and the two-sample t-test or Wilcoxon rank-sum test for intergroup comparison were performed for continuous variables. Chi-square test or Fisher’s exact test was used for categorical variables. Medication adherence to the study agent was calculated as (number of medications/planned number of medications during the observation period) × 100 (%). In the case of discontinuation or dropout, the planned number of medications until discontinuation or dropout was used as the denominator. A two-sample t-test was performed for the intergroup comparison of medication adherence. Medication preference was asked of the study patients at three levels (prefer once-weekly agent, prefer daily agent, or either). The number and proportion of participants at each level were calculated, and Bowker’s test of symmetry for intragroup comparisons and chi-square tests for intergroup comparisons were performed. To determine the frequency of adverse events, Fisher’s exact test was used for intergroup comparisons. Correlation analysis was performed using Pearson’s and Spearman’s rank correlation coefficients. To explore the background characteristics associated with improvement in DTBQ, logistic regression analysis was performed with improvement of DTBQ total score (change in DTBQ total score from baseline to week 12 is < 0) as a response variable and background characteristics as explanatory variables. The SAS statistical software package version 9.4 (SAS, Cary, NC, USA) was used to perform all statistical analyses. To avoid bias and ensure quality, data collection, management and statistical analyses were performed by third-party entities (Soiken Inc., Osaka, Japan).
Results
Baseline Characteristics of Study Participants
A total of 367 potential participants were screened, and 151 were excluded from the study. Of the 151 excluded participants, 115 did not meet the eligibility criteria, 25 did not provide consent to participate, and 11 were not registered because the planned number of participants had been registered prior to their registration. Finally, 216 participants were registered and randomly categorized to the treatment groups (Fig. 1). Of these, 109 patients were assigned to the group that switched to omarigliptin and 107 were assigned to the group that continued daily DPP4is.
The baseline characteristics of the registered participants were well balanced between the groups, except for the blood glucose level, comorbidity of dyslipidemia and number of medications per day (Table 1).
Change in Burden of Pharmacotherapy
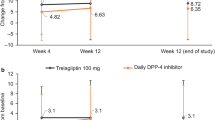
The DTBQ total score significantly decreased from baseline to week 12 in both groups; however, no significant intergroup difference was observed (Table 2). Among the three DTBQ subscales, the implementation and flexibility burden scores significantly decreased in the group that switched to omarigliptin, although no significant intergroup differences were observed.
In each item of the DTBQ among the 18 questions, scores for items 1, 2, 6, 7, 11, 12, and 13 showed a significant intragroup decrease, and scores for item 14 showed a significant increase in the group that switched to omarigliptin (Table 3). Items 6 and 7 significantly decreased or tended to decrease in the group that switched to omarigliptin compared with the group that continued daily DPP4is. Meanwhile, items 14 and 15 significantly increased in the group that switched to omarigliptin compared with the group that continued daily DPP4is.
When stratified by medication preference at baseline, in patients who preferred a once-weekly agent at baseline, the DTBQ total score as well as implementation and flexibility burden scores significantly decreased in the group that switched to omarigliptin, and in patients who preferred either. Meanwhile, the flexibility burden score significantly decreased while the DTBQ total score and implementation burden score numerically decreased in the group that switched to omarigliptin (Table 4).
Medication Preference and Adherence
Medication preferences differed significantly between the baseline and week 12 (Table 5). At baseline, the proportion of patients who preferred once-weekly agents was higher in the group that switched to omarigliptin than in the group that continued daily DPP4is, and the proportion of patients who preferred daily agents was higher in the group that continued daily DPP4is than in the group that switched to omarigliptin. At week 12, the medication preference for the assigned study agent increased in both groups. Medication adherence was as high as 97% in both groups without significant intergroup differences.
Glucose Metabolism Biomarkers
HbA1c and fasting blood glucose levels significantly increased in the group that switched to omarigliptin compared to the group that continued daily DPP4is (Table 6).
Factors Associated with Change in DTBQ Scores
The DTBQ total score and subscale scores at baseline were significantly associated with the improvement in DTBQ (change in DTBQ total score from baseline to week 12 was < 0), with an odds ratio > 1, indicating that higher DTBQ scores at baseline were associated with a higher proportion of patients whose DTBQ scores improved from baseline to week 12 (Table 7). In particular, the DTBQ total score and all subscale scores at baseline were significantly associated with improvement in DTBQ in all registered participants or in the group that switched to omarigliptin, whereas only the flexibility burden score at baseline was significantly associated with improvement in DTBQ in the group that continued daily DPP4is.
Discussion
This ONWARD-DPP4 study was the first prospective randomized controlled trial to compare the impact of switching from daily DPP4is to omarigliptin, a once-weekly DPP4i, with continuing daily DPP4is, on patients’ QOL, especially in terms of the burden of pharmacotherapy. The primary endpoint of this study, the change in the DTBQ total score from baseline to week 12, did not differ significantly between the groups. To calculate the target sample size, change in DTBQ total score from baseline to week 12 was assumed as − 10.5 and 0.0 in the group that switched to omarigliptin and the group that continued daily DPP4is, respectively. However, the actual change in DTBQ total score from baseline to week 12 in this study was − 4.0 and − 2.0 in the group that switched to omarigliptin and the group that continued daily DPP4is, respectively. Since the change in the DTBQ total score in the group that switched to omarigliptin was less than half of the assumption and a significant intragroup decrease in the DTBQ total score was observed in the group that continued daily DPP4is, a significant intergroup difference in the primary endpoint was not observed in this study. In addition, although this study employed HbA1c and age as allocation factors, the baseline characteristics of the participants were significantly different between the groups in blood glucose level, comorbidity of dyslipidemia and number of medications per day and tended to be different in comorbidity of hypertension. Further studies are required with target sample size calculated by the mean change and standard deviation obtained in this study and considering the allocation factors by adding the number of medications per day, for example.
The implementation and flexibility burden scores, as well as the item scores 1, 2, 6 and 7, which comprise the implementation burden score and 11–13, which comprise the flexibility burden score, were significantly improved in the group that switched to omarigliptin, suggesting a decrease in the treatment burden. Compared with the group that continued daily DPP4is, significantly increased (worsened) score was observed in items 14 and 15, which asked about the burden on glycemic control, and significantly higher changes were observed in the group that switched to omarigliptin. These findings were consistent with the significant increase in HbA1c and fasting blood glucose levels in the group that switched to omarigliptin, although the respective values were 0.1% and 5.8 mg/dl, which seemed not to be clinically meaningful. Nevertheless, attending physicians should pay attention to the worsening of the glycemic control and patients’ treatment burden upon switching from daily DPP4is to omarigliptin.
Based on the results of this study, DTBQ and medication preference can be considered in determining pharmaceutical strategy for type 2 diabetes mellitus. Since higher DTBQ scores at baseline were associated with improvement in DTBQ (Table 7), and the DTBQ scores were improved in patients who preferred once-weekly agents at baseline (Table 4), once-weekly agents may be considered in patients whose DTBQ is high and who prefer once-weekly agent. Once-weekly agents can be an option in patients whose DTBQ is high but prefer daily agent or in patients whose DTBQ is low but prefer once-weekly agents. In contrast, continuation of daily agents may be considered in patients with low DTBQ scores and who prefer daily agents.
This study has several limitations. First, this was an open-label study that lacked the blinding of both patients and physicians, and the primary endpoint was PRO. This may have caused a bias in the results of this study. In addition, the DTBQ total score significantly decreased from baseline to week 12 even in the group that continued the daily DPP4is. This may also be one of the biases of the open-label nature of PRO. Second, all patients in this study were Japanese, and weekly DPP4is, omarigliptin and trelagliptin were launched in Japan, but not in the US and Europe. Therefore, the generalizability of the results in this study to other countries or patients of other ethnicities is unknown. Third, although this study enrolled patients with type 2 diabetes mellitus who were taking one of the daily DPP4is, the number of medications per day of other OHAs or medical agents for other chronic illnesses were not restricted, because we aimed to assess the effect of omarigliptin on patients’ psychological attitudes and responses in the real-world situation. Since the mean of medication tablets per day and the mean of medication tablets of OHAs per day in the group that switched to omarigliptin were 6.8 and 3.8, respectively, it might be overwhelming to feel the decrease in the burden of medication by switching of only a tablet from daily to weekly. Further investigations are required considering the total number of medications.
Conclusion
Although this study failed to demonstrate the improvement of DTBQ total score by switching from daily DPP4is to omarigliptin compared with continuing the daily DPP4is; DTBQ subscale scores, implementation and flexibility burden score were significantly improved only in the group that switched to omarigliptin, suggesting the possibility of switching from daily DPP4is to omarigliptin to decrease the patients’ burden on medication.
References
Scheen AJ. Safety of dipeptidyl peptidase-4 inhibitors for treating type 2 diabetes. Expert Opin Drug Saf. 2015;14:505–24. https://doi.org/10.1517/14740338.2015.1006625.
Tella SH, Rendell MS. DPP-4 inhibitors: focus on safety. Expert Opin Drug Saf. 2015;14:127–40. https://doi.org/10.1517/14740338.2015.977863.
Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708. https://doi.org/10.1007/s00125-012-2827-3.
Fukuda M, Doi K, Sugawara M, Mochizuki K. Efficacy and safety of sitagliptin in elderly patients with type 2 diabetes mellitus: a focus on hypoglycemia. J Diabetes Investig. 2019;10:383–91. https://doi.org/10.1111/jdi.12915.
Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(1):102–9. https://doi.org/10.1111/jdi.12490.
Bouchi R, Sugiyama T, Goto A, Imai K, Ihana-Sugiyama N, Ohsugi M, et al. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J Diabetes Investig. 2022;13:280–91. https://doi.org/10.1111/jdi.13636.
Japan diabetes society. Treatment guide for diabetes 2016–2017. Tokyo: Bunkodo; 2016.
Japan diabetes society. Treatment guide for diabetes 2018–2019. Tokyo: Bunkodo; 2018.
Japan diabetes society. Japanese clinical practice guideline for diabetes 2022–2023. Tokyo: Bunkodo; 2023.
Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in Type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;2022(45):2753–86. https://doi.org/10.2337/dci22-0034.
Burkhart PV, Sabaté E. Adherence to long term therapies. Evidence for action. J Nurs Scholarsh. 2003;35:207. https://apps.who.int/iris/handle/10665/42682
U.K. Prospective diabetes study group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65. https://doi.org/10.1016/S0140-6736(98)07037-8.
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in Type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–701. https://doi.org/10.2337/dci18-0033.
McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1040–3. https://doi.org/10.1111/dom.13160.
UK Prospective Diabetes Study Group. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). UK prospective diabetes study group. Diabetes Care. 1999;22:1125–36. https://doi.org/10.2337/diacare.22.7.1125.
Shim YT, Lee J, Toh MP, Tang WE, Ko Y. Health-related quality of life and glycaemic control in patients with type 2 diabetes mellitus in Singapore. Diabet Med. 2012;29:e241–8. https://doi.org/10.1111/j.1464-5491.2012.03689.x.
Pharmaceuticals and Medical Devices Agency in Japan (2020) Package insert of MARIZEV tablets 12.5 mg and 25 mg. Available at. https://www.info.pmda.go.jp/go/pack/3969025F1022_1_10/
Krishna R, Addy C, Tatosian D, Glasgow XS, Gendrano IN, Robberechts M, et al. Pharmacokinetics and pharmacodynamics of omarigliptin, a once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, after single and multiple doses in healthy subjects. J Clin Pharmacol. 2016;56:1528–37. https://doi.org/10.1002/jcph.773.
Tsuchiya S, Friedman E, Addy C, Wakana A, Tatosian D, Matsumoto Y, et al. Single and multiple dose pharmacokinetics and pharmacodynamics of omarigliptin, a novel, once-weekly dipeptidyl peptidase-4 inhibitor, in healthy Japanese men. J Diabetes Investig. 2017;8:84–92. https://doi.org/10.1111/jdi.12538.
Gantz I, Okamoto T, Ito Y, Okuyama K, O’Neill EA, Kaufman KD, et al. A randomized, placebo- and sitagliptin-controlled trial of the safety and efficacy of omarigliptin, a once-weekly dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:1602–9. https://doi.org/10.1111/dom.12988.
Reaney M, Yu M, Lakshmanan M, Pechtner V, van Brunt K. Treatment satisfaction in people with type 2 diabetes mellitus treated with once-weekly dulaglutide: data from the AWARD-1 and AWARD-3 clinical trials. Diabetes Obes Metab. 2015;17:896–903. https://doi.org/10.1111/dom.12527.
Ishii H, Shin K, Tosaki T, Haga T, Nakajima Y, Shiraiwa T, et al. Reproducibility and validity of a questionnaire measuring treatment burden on patients with Type 2 diabetes: Diabetic Treatment Burden Questionnaire (DTBQ). Diabetes Ther. 2018;9:1001–19. https://doi.org/10.1007/s13300-018-0414-4.
Acknowledgements
The authors thank all the clinical staff for their assistance in the execution of the study and thank all study participants.
Funding
This study was financially supported by Kissei Pharmaceutical Co., Ltd. The journal’s Rapid Service Fee, the fee for technical assistance in the launch and execution of the study, and the medical writing of the manuscript by Soiken, Inc. were also financially supported by Kissei Pharmaceutical Co., Ltd.
Medical Writing, Editorial and Other Assistance
The authors thank Soiken Inc. for their technical assistance in the launch and execution of the study and Arata Yoneda in Soiken Inc. for his support in the medical writing of the manuscript. The research fund provided by Kissei Pharmaceutical Co., Ltd., covered the fees for technical assistance and medical writing.
Author Contributions
Hiroshi Ishii contributed to the conception and design of the study, development and amendment of the study protocol, subject enrollment, study implementation, data collection and writing of the article. Nozomu Kamei, Dai Shimono and Takahiro Tosaki contributed to the study design, subject enrollment, study implementation and data collection. Toru Kitazawa, Daisuke Suzuki, Yutaka Wakasa, Hiroaki Seino, Mariko Oishi, Hiroshi Ohashi and Kenshi Higami contributed to participant enrollment, study implementation and data collection. Hiroaki Akai supervised the conception, design and implementation of the study. All the authors have read and approved the final version of the manuscript.
Disclosures
Hitoshi Ishii received research funding from Kissei Pharmaceutical Co., Ltd., and speaker’s bureaus from Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., MSD K.K. and Sumitomo Pharma Co., Ltd. Nozomu Kamei received a speaker’s bureau fee from Eli Lilly Japan K.K. and Sumitomo Pharma Co. Ltd. Dai Shimono received a speaker’s bureau from Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Sumitomo Pharma Co. Ltd., Novo Nordisk Pharma Ltd. and Nippon Boehringer Ingelheim Co., Ltd. Takahiro Tosaki received research funds from Novo Nordisk Pharma Ltd. and Eli Lilly Japan K.K., and speaker’s bureau from Novo Nordisk Pharma Ltd., Sumitomo Pharma Co. Ltd. and Eli Lilly Japan K.K. Toru Kitazawa received speaker’s bureau from Sanofi K.K., Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Company, Limited, Abbott Medical Japan LLC., Sanwa Kagaku Kenkyusho Co., Ltd., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Sumitomo Pharma Co., Ltd., AstraZeneca K.K., Kowa Company, Ltd., Eli Lilly Japan K.K., and Kyowa Kirin Co., Ltd. Hiroaki Seino received research funds from Novo Nordisk Pharma Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., and YL Biologics Limited, and Sanofi K.K. Hiroaki Akai received the speaker’s bureau from Sumitomo Pharma Co., Ltd. Hiroaki Akai also had leadership in the Japan Association for Diabetes Education and Care, Certification Commission for Diabetes Educators in Miyagi, Certification Board for Diabetes Educators in Japan and Promotion Council for Diabetes Prevention and Countermeasures in Miyagi. Tetsuji Niiya, Daisuke Suzuki, Yutaka Wakasa, Mariko Oishi, Hiroshi Ohashi and Kenshi Higami declare no potential conflicts of interest.
Compliance with Ethics Guidelines
This study including its protocols and all participating medical institutions were inspected and approved by the Japan Physicians Association Clinical Research Review Board, which is certified by the Minister of Health, Labour and Welfare in Japan, according to the requirements of the Clinical Trials Act. This study was registered in the Japan Registry of Clinical Trials (jRCT) (Registration No. jRCTs031200437) after receiving approval from a certified review board, according to the requirements of the Clinical Trials Act. The study was conducted in accordance with the Declaration of Helsinki, Clinical Trials Act and other current legal regulations in Japan. Written informed consent was obtained from all enrolled patients who met the eligibility criteria before treatment.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available because of the lack of a statement in the study protocol, which enables data sharing with a third party after the end of the study, and in the informed consent documents, as well as a lack of approval for data sharing by the Japan Physicians Association Clinical Research Review Board.
Author information
Authors and Affiliations
Consortia
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ishii, H., Kamei, N., Shimono, D. et al. Treatment Burden on Once-Weekly Omarigliptin Versus Daily Dipeptidyl Peptidase-4 Inhibitors in Patients with Type 2 Diabetes: Randomized Controlled Trial (ONWARD-DPP4 Study). Diabetes Ther 14, 1639–1658 (2023). https://doi.org/10.1007/s13300-023-01442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01442-0