Abstract
Introduction
The efficacy of iGlarLixi, a fixed-ratio combination of basal insulin glargine 100 units/mL (iGlar) and the short-acting GLP-1 RA lixisenatide (Lixi), was established in people with type 2 diabetes (T2D) who were advancing therapy from oral antidiabetic drugs (OADs) and basal insulin (BI). This retrospective study aimed to evaluate the effectiveness and safety of iGlarLixi using real-world data from people with T2D in the Adriatic region countries.
Methods
This was a non-interventional, retrospective, multicenter, cohort study with the collection of pre-existing data at iGlarLixi initiation and after 6 months of treatment in real-world clinical and ambulatory settings. The primary outcome was the change in glycated hemoglobin (HbA1c) at 6 months after iGlarLixi initiation. Key secondary outcomes included the proportion of people achieving HbA1c < 7.0%, the effect of iGlarLixi on fasting plasma glucose (FPG), body weight and body mass index (BMI).
Results
In this study, 262 participants (130 in Bosnia and Herzegovina, 72 in Croatia and 60 in Slovenia) initiated treatment with iGlarLixi. The participants had a mean ± SD age of 66.2 ± 7.9 years and the majority were women (58.0%). The mean baseline HbA1c was 8.9 ± 1.7% and the mean body weight was 94.3 ± 18.0 kg. After 6 months of treatment, the reduction in the mean HbA1c was statistically significant (1.11 ± 1.61%, 95% confidence internal [CI] 0.92, 1.31; p < 0.001), and the proportion of participants who achieved HbA1c < 7.0% had significantly increased from baseline (8.0–26.0%, p < 0.001). The change in mean FPG (mmol/L) levels was significant (2.7 ± 4.4 [95% CI 2.1, 3.2; p < 0.001]). The mean ± SD body weight and BMI were significantly reduced by 2.9 ± 4.3 kg (95% CI 2.3, 3.4; p < 0.001) and 1.3 ± 4.4 kg/m2 (95% CI 0.7, 1.8; p < 0.001), respectively. Two serious hypoglycemia episodes and one adverse gastrointestinal effect (nausea) were registered.
Conclusions
This real-world study demonstrated the effectiveness of iGlarLixi for improving glycemic control and decreasing body weight in people with T2D who need to advance therapy from OADs or insulin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The efficacy of iGlarLixi, a fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide, in people with type 2 diabetes (T2D) who were suboptimally controlled on oral antidiabetic drugs (OADs) or basal insulin has been previously reported. However, real-world evidence on the effectiveness of iGlarLixi in a diverse population such as in people from the Adriatic region is limited. |
This study aimed to evaluate the effectiveness and safety of iGlarLixi using real-world data from people with T2D from Bosnia and Herzegovina, Croatia and Slovenia. |
What was learned from the study? |
Treatment with iGlarLixi for 6 months resulted in significant reductions in glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), body weight (BW) and body mass index (BMI). The proportion of people who achieved HbA1c < 7.0% was significantly increased, but further strategies must be taken into consideration to increase the number of patients who are on glycemic target. |
iGlarLixi is a favorable therapeutic option for people with suboptimally controlled T2D who need to advance therapy from OADs or insulin (including premix insulins), and it could enable more people to achieve improved glycemic control with a low risk of hypoglycemia and provide a weight benefit. |
Introduction
Glycemic control, essential to the management of people living with type 2 diabetes (T2D), requires progressive and timely treatment advancement, often leading to the need for insulin therapy [1]. However, therapeutic advancement and insulin initiation are often delayed, and when finally begun, the improvements in glycemic control are typically accompanied by weight gain and an increased incidence of hypoglycemia [2]. In people with T2D, therapeutic inertia during initiation or intensification and suboptimal glycemic control may contribute to a poor quality of life, the onset of diabetes-related complications and mortality [3, 4].
Global and regional consensuses or guidelines on diabetes management recommend initiating glucagon-like peptide-1 receptor agonists (GLP-1RAs) as the first injectable therapy and intensifying with a combination injectable therapy (insulin plus GLP-1RAs) if the glycated hemoglobin (HbA1c) remains above the target [5, 6]. Fixed-ratio combinations (FRCs) of basal insulin and GLP-1RA have demonstrated effective glucose lowering and mitigation of weight gain with no increased risk of hypoglycemia in various large, randomized trials compared to basal insulin (BI) [7,8,9,10,11,12,13,14,15]. iGlarLixi, a once-daily titratable FRC of basal insulin glargine 100 U/mL (iGlar) and the short-acting GLP-1RA lixisenatide (Lixi) [16], has demonstrated the ability to significantly improve glycemic control with no increase in hypoglycemia risk compared to iGlar and low levels of gastrointestinal adverse events compared to Lixi in studies conducted in people with long-standing T2D [8, 9].
Findings regarding the efficacy and safety of iGlarLixi are consistent regardless of age, diabetes duration, and baseline HbA1c [8,9,10]. The glucose-lowering effect, considerable safety profile, and once-daily administration schedule of iGlarLixi make it a valuable treatment option for people with T2D requiring treatment advancement [17]. Although the efficacy and safety of iGlarLixi has been established in various randomized trials, data from the real-world setting with a more diverse population may be of added value for clinicians’ prescribing decisions.
This retrospective study aims to evaluate the effectiveness and safety of iGlarLixi in people with T2D in a real-world setting in Adriatic region countries such as Slovenia, Croatia and Bosnia and Herzegovina.
Methods
Study Design
ENSURE-ADR was a non-interventional, retrospective, multicenter, cohort study performed in real-world clinical and ambulatory settings in Slovenia, Croatia, and Bosnia and Herzegovina (B&H). Retrospective data were collected from the index date and baseline period prior to iGlarLixi initiation from the medical records. The index date was the date of initiation of iGlarLixi treatment. The inclusion visit was defined as the date when signed informed consent was provided by the participants who were on iGlarLixi treatment for at least 6 months (Fig. 1). Adults with T2D who were on iGlarLixi treatment for at least 6 months from the index date but may or might not have continued iGlarLixi at the study inclusion visit were included in this study. Medical records of the included participants had data on HbA1c, fasting plasma glucose values, weight, and insulin dose available at the time of iGlarLixi initiation and 6 months (± 45 days) after the start of iGlarLixi. People who were < 18 years of age, who were diagnosed with type 1 diabetes, who had previously participated in any clinical trial on iGlarLixi or had administered an experimental drug during the 6-month observation period, who had severe comorbidities such as renal insufficiency requiring hemodialysis, who were undergoing active cancer treatment, who had a major surgery during the 6-month observation period, or who were pregnant women were excluded. Treatment with iGlarLixi was decided upon by the treating physician according to the summary of product characteristics of Suliqua® and the reimbursement rules in the participating country.
This study was conducted in accordance with the local legislation and the international guidelines and was approved by the regulatory authorities and the ethics committees in each of the three countries (Central Ethics Committee [CEC] of the Agency for Medicinal Products and Medical Devices of Croatia, Croatia; The Commission of the Republic of Slovenia for Medical Ethics, Slovenia; Ethics Committee of the Clinical Center of Banja Luka, Bosnia and Herzegovina). Informed consent was obtained from all individual participants included in the study who were on initiated iGlarLixi therapy for at least 6 months. All monitoring activities were conducted in accordance with the International Conference on Harmonization/Good Clinical Practice where applicable, and Good Pharmacoepidemiology Practices were followed.
Study Outcomes
The primary outcome of this study was the change in HbA1c 6 months after iGlarLixi initiation. Secondary outcomes included the proportion of participants achieving HbA1c < 7%, the proportions of participants with a weight loss, a weight increase, and no weight change among those with HbA1c < 7% and HbA1c ≥ 7%, the effect of iGlarLixi on fasting plasma glucose, body weight and body mass index (BMI) in the overall population, the proportion of participants injecting iGlarLixi before breakfast/lunch/dinner, reasons for initiating iGlarLixi (data from an ad hoc survey questionnaire of physicians), and the proportion of participants who switched to iGlarLixi from different previous treatments. Additionally, the baseline characteristics that were associated with a change in HbA1c, FPG, body weight, and BMI after 6 months of treatment were determined.
Statistical Methodology
All statistical analyses were based on the baseline value, defined as the last available value prior to the iGlarLixi initiation, and the values at the end of the 6-month follow-up period (inclusion visit). Continuous data were presented as mean values with standard deviations (SD) and 95% confidence intervals (CIs) for means, and categorical data were presented as absolute numbers and percentages.
Depending on the type and distribution of the examined parameter, the chi-square test, the Mantel–Haenszel test, Student’s t test for independent or paired samples, the Mann–Whitney test (not for a Gaussian distribution), the Wilcoxon signed ranks test, or ANOVA was used. In all tests, an alpha level of 0.05 (p < 0.05) was considered statistically significant. Predictor analyses for achieving HbA1c < 7% were based on binary logistic regression, whereas a linear regression model was used for the change in the change in HbA1c in the follow-up period.
The sample size was determined based on the expected mean change in HbA1c over 6 months of treatment. Assuming a standard deviation of 0.9% and a mean HbA1c reduction of 0.3%, a sample size of 260 would result in 95% CI values ranging from 0.21 to 0.39%. A margin of a 0.3% reduction in HbA1c was selected, as apparently small reductions in HbA1c could be clinically relevant in terms of a reduction in diabetes-related complications and are recommended in the guideline on the clinical investigation of medicinal products for the treatment or prevention of diabetes [18].
Results
Demographics and Clinical Characteristics
A total of 262 people with T2D were included in the study, of whom 130 (49.6%) were from Bosnia and Herzegovina, 72 (27.5%) were from Croatia, and 60 (22.9%) were from Slovenia. The mean ± SD age of the overall population was 66.2 ± 7.9 years and the majority were females (58.0%). The majority of the participants (80.0%) were obese, contributing to a body mass index (BMI) of 33.5 ± 6.4 kg/m2. The duration of diabetes was 16.0 ± 7.9 years and the baseline HbA1c was 8.9 ± 1.7%, while FPG was 10.7 ± 4.3 mmol/L and self-monitored plasma glucose (SMPG) was 9.7 ± 3.6 mmol/L (Table 1). Prior to iGlarLixi initiation, 74.0% of the participants received insulin (± OAD) therapy and about 86% had received treatment with OADs, of which metformin was the most commonly used (78.6%), followed by sulfonylureas (30.8%).
Concomitant Insulin and Other Glucose-Lowering Therapies
After iGlarLixi initiation, metformin was the most common concomitant OAD used in 76.3% participants. Sulfonylureas (SU) were discontinued in more than half of the participants but were still used in 13.4% of participants after initiating iGlarLixi, while insulin was mostly discontinued after iGlarLixi initiation, with 22 participants continuing insulin therapy, of whom 17 were mainly using bolus insulin (Supplementary Fig. 1).
Change in Glycated Hemoglobin
Mean HbA1c decreased from 8.99 ± 1.69% at baseline to 7.85 ± 1.35% at 6 months after iGlarLixi initiation (primary outcome), with a mean change from baseline of 1.11 ± 1.61% (95% CI 0.92, 1.31; p < 0.001) (Fig. 2a).
a HbA1c and b the proportion of participants with HbA1c < 7.0% at baseline and 6 months after iGlarLixi initiation. c The pattern of weight change among participants with HbA1c < or ≥ 7%. The baseline was the value available on or before the index date. HbA1c glycated hemoglobin, iGlarLixi insulin glargine 100 U/mL and lixisenatide, NS not significant, SD standard deviation
The reduction in HbA1c was significant across all baseline subgroups (p < 0.01) except for the subgroup with baseline HbA1c < 7.0%, the subgroup with previous use of fast-acting insulins or analogs (intermediate- or long-acting) combined with fast-acting insulin analogs, or participants who had iGlarLixi before dinner (Supplementary Table 1).
The proportion of participants who had achieved HbA1c < 7.0% significantly increased after 6 months of iGlarLixi treatment (from 8.0 to 26.0%, p < 0.001) (Fig. 2b). No significant differences were observed in the proportions of participants with weight loss, weight increase and no weight change between the groups with HbA1c < 7% and HbA1c ≥ 7% (Fig. 2c).
Changes in FPG and SMPG
After 6 months of treatment, the mean FPG and SMPG were significantly reduced by 2.7 ± 4.4 (95% CI 2.1, 3.2, p < 0.001) and 2.1 ± 3.3 mmol/L (95% CI 1.4, 2.7, p < 0.001), respectively (Fig. 3a).
Changes from baseline to 6 months of iGlarLixi initiation in a FPG (mmol/L) and SMPGs (mmol/L) and b body weight (kg) and BMI (kg/m2). The baseline was the value available on or before the index date. CI confidence interval, FPG fasting plasma glucose, iGlarLixi insulin glargine 100 U/mL and lixisenatide, SD standard deviation, SMPG self-monitored plasma glucose
The reduction in FPG was significant across all baseline subgroups except for those with baseline HbA1c < 7.0% and those with previous use of fast-acting insulins, sodium-glucose cotransporter-2 inhibitor (SGLT-2i) or GLP-1RA + insulin (Supplementary Table 1).
Effects of iGlarLixi on Body Weight and Body Mass Index
The decrease in mean (± SD) body weight from baseline (94.1 ± 17.4 kg) to 6 months post-treatment was significant with iGlarLixi (91.1 ± 17.2 kg), with a reduction of 2.9 ± 4.3 kg (95% CI 2.3, 3.4; p < 0.001). Similarly, a significant reduction in BMI from baseline (33.5 ± 6.4 kg/m2) to 6 months post-treatment with iGlarLixi (32.2 ± 5.0 kg/m2) was observed, with a reduction of 1.3 ± 4.4 kg/m2 (95% CI 0.7, 1.8; p < 0.001) (Fig. 3b). Reductions in body weight and BMI were registered in all baseline subgroups except for the participants who were using GLP1-RA (and who were on SGLT-2is to change the BMI) before switching to iGlarLixi, were previously using insulins or intermediate- or long-acting combined with fast-acting insulin analogs, and were administering iGlarLixi before dinner (Supplementary Table 2).
Time of Administration and iGlarLixi Pen Type
A majority of the participants at initiation as well as after 6 months of treatment administered iGlarLixi before breakfast (63.7% and 62.6%), and there were no significant changes from baseline to 6 months after in the proportion of participants who injected iGlarLixi before breakfast/lunch/dinner (Supplementary Fig. 2).
At initiation, about 67% of the participants used iGlarLixi 100U/mL + 50 µg/mL (Suliqua® 2:1 pen [FRC of 2 U iGlar and 1 µg Lixi]). While the proportion of the participants who used iGlarLixi 100U/mL + 33 µg/mL (Suliqua® 3:1 pen [FRC of 3 U iGlar and 1 µg Lixi]) significantly increased over the 6 months, those who used the Suliqua® 2:1 pen significantly decreased from initiation to after 6 months (Fig. 4). The participating physicians reported that 82.8% of the participants were satisfied with the way titration was performed.
Reasons for Initiating iGlarLixi
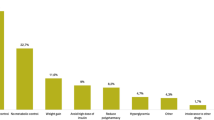
The most common reason provided by the participating physicians for initiating iGlarLixi was better glycemic control (64.5%), followed by weight benefit (45.8%) and efficacy (43.1%). The most common combinations among the reasons given by participating physicians for initiating iGlarLixi were “better control, better adherence, efficacy” (8.8%), “better control, efficacy, less variability” (7.3%), and better “control, efficacy, weight benefit” (7.3%). All the reasons and the combinations of reasons are presented in detail in Table 2.
Adverse Events
Two participants each experienced one serious hypoglycemic episode (blood glucose level < 54 mg/dL [3.0 mmol/L]) and required the assistance of another person or other resuscitative actions, which corresponded to 0.015 severe hypoglycemia episodes per patient/year. One adverse gastrointestinal event (nausea) was registered.
Discussion
In the ENSURE study, a significant overall improvement in glycemic control after 6 months from iGlarLixi initiation was demonstrated, with significant reductions in HbA1c, FPG and SMPG along with decreases in body weight and BMI. The reduction in HbA1c was achieved across groups that used different concomitant glucose-lowering therapies, except for fast-acting insulins or analogs (intermediate- or long-acting) combined with fast-acting insulin analogs.
National guidelines on diabetes management generally align with the European guidelines; in particular, the Croatian Guidelines for the Pharmacotherapy of Type 2 Diabetes recommends the use of GLP-1 RA or insulin in combination with metformin in people who have suboptimal glycemic control after 3 months with metformin alone. Insulin therapy alone or in combination with other drugs is recommended in people who are newly diagnosed with diabetes and have HbA1c ≥ 10%, whereas GLP-1 RA + basal insulin is recommended for those who have excessive body weight and suboptimally controlled glycemia [6].
Among the OADs, metformin and SUs are the most widely used for diabetes management. Guidelines recommend that the dose of SUs should be reduced or that they should be stopped in patients initiating insulin therapy [5]. In the ENSURE study, about 13% of the participants still used SUs after initiating iGlarLixi. This may be because of the need to treat hyperglycemia aggressively in some patients or due to limited access to HCPs for dose and medication adjustment, particularly during the COVID period. As this was a retrospective real-world study, no recommendations were provided on the concomitant use of medications. Moreover, few participants continued insulin therapy after iGlarLixi initiation.
Previous randomized controlled trials (RCTs) have reported the efficacy and safety of iGlarLixi in people with T2D [8,9,10]. Results from the iGlarLixi arm of these RCTs are comparable with the results observed in the present study; however, when comparing results across studies, the differences in the design and population should be carefully considered. The participants who were enrolled in the ENSURE study were older, had a longer duration of diabetes, and had a higher BMI and body weight at baseline than the cohorts in the LixiLan-O [8] and LixiLan-L [9] studies. The mean HbA1c and FPG value at baseline in the ENSURE population were higher when compared with the cohorts in the LixiLan-O, LixiLan-L and LixiLan-G studies [8,9,10], indicating a potentially greater cardiovascular risk and the common presence of late diabetes complications in the ENSURE study [19].
The reduction in HbA1c observed in this study (1.1%) was similar to that for the iGlarLixi arm in the LixiLan-L study (1.1%) after 6 months [9]. The reduction in HbA1c in the LixiLan-O study (1.6%) was higher when compared to that observed in the ENSURE study [8], in which only 24.8% of participants had been switched from OADs without insulin therapy. In this study, the proportion of participants who reached HbA1c < 7.0% significantly increased after 6 months of iGlarLixi initiation; however, this percentage was lower (26.0%) than those reported in the LixiLan-O (73.7%), LixiLan-L (54.9%) and LixiLan-G (61.9%) studies [8,9,10] and consistent with those in other real-world studies conducted in the European region [20, 21]. This considerable difference could be partly because of the higher mean baseline HbA1c level in the ENSURE study population: about 42% of the participants had HbA1c ≥ 9% at baseline. Moreover, dose titration is generally lower in the real-world setting compared to that in treat-to target RCTs, contributing to the lower achievement of the HbA1c target. A multifactorial approach including timely and optimal titration of FRC along with patient support/diabetes education and adherence to recommended lifestyle modifications and treatment could enable people with suboptimally controlled hyperglycemia to achieve their glycemic targets.
The mean reduction in FPG in the ENSURE study was greater than those recorded in the iGlarLixi cohorts of other RCTs (2.7 mmol/L versus 0.4 mmol/L in LixiLan-L and 2.1 mmol/L in LixiLan-G) [9, 10] and in a real-world study (2.1 mmol/L) [20], despite the higher mean baseline FPG in the ENSURE population. The reduction was smaller when compared to that reported in the LixiLan-O study (3.5 mmol/L) [8]. Though the reduction in FPG in the overall population of the STAR.Ro study (3.1 mmol/L) was greater than that reported in the current study, people who were previously treated with basal insulin reported a smaller mean reduction of 2.3 mmol/L [21]. At the end of 6 months of treatment, both HbA1c and FPG levels were significantly reduced when iGlarLixi was administered before breakfast or before lunch. Reductions in HbA1c and FPG were also observed when iGlarLixi was administered before dinner. However, this reduction did not reach statistical significance due to the very low proportion (n/N = 7/261) of participants in this group.
The proportion of participants recorded with a decrease in body weight was almost three-quarters and is higher than that reported in the LixiLan studies [8,9,10]. The observed decreases in body weight and BMI were significant in the overall population and across all baseline subgroups except in the 15.2% of participants who were treated with GLP1-RA before switching to iGlarLixi. In the LixiLan-G study, body weight increased by approximately 1.9 kg in the iGlarLixi group and decreased by approximately 1.1 kg in the GLP-1RA group [10]. Despite the participants in the ENSURE study being elderly (> 65 years of age) at baseline, with a higher HbA1c and a longer duration of diabetes, iGlarLixi was associated with a weight benefit (− 2.3 kg) in the present study compared to the iGlarLixi arm of the LixiLan-G study. The decrease in body weight observed in the ENSURE study was greater than in that reported at week 24 of the STAR.Ro real-world study (1.6 kg) and was similar to that reported in another real-world study (2.32 kg) [19, 21].
Premix insulin remains frequently used, either as the initial injectable therapy or as an intensifying therapy from basal insulin, and as the ENSURE study was conducted in a real-world setup, about 25% of the total population were previously treated with insulins, including premixed insulin. Those participants who switched from premix regimens to iGlarLixi showed improved glycemic parameters (HbA1c, FPG, and SMPG) along with decreases in body weight and BMI. iGlarLixi 100 U/mL + 50 µg/mL is recommended when initiating iGlarLixi treatment for people on OAD or on insulin therapy below 30 U [22]. In the present study, the majority of the participants (67.2%) initiated iGlarLixi treatment with the 100 U/mL + 50 µg/mL pen. There was a significant increase in the proportion of participants using the 100 U/mL + 33 µg/mL pen and a decrease in 100 U/mL + 50 µg/mL pen use 6 months after iGlarLixi initiation, suggesting that the titration process was well performed, especially while switching from the iGlarLixi 100 U/mL + 50 µg/mL (2:1) pen to the 100 U/mL + 33 µg/mL (3:1) pen. Additionally, the participants who switched to iGlarLixi 100 U/mL + 33 µg/mL were likely those who required more insulin based on their FPG. In line with this, most of the physicians had the opinion that the titration was performed satisfactorily.
In terms of overall safety and adverse drug events, the proportion of people with at least one severe symptomatic hypoglycemia (blood glucose level < 54 mg/dL and requiring the assistance of another person or other resuscitative actions) event was low, despite significant reductions in FPG and clinically meaningful improvements in HbA1c. Two participants (0.8%) experienced one hypoglycemia event each, which corresponds to 0.015 severe hypoglycemia events per person-year, and the only reported adverse drug reaction in this study was nausea. Based on these observations, the safety profile of this study was in line with that reported in the LixiLan-L RCT [8]. Similarly, one episode of severe hypoglycemia was reported in the STAR.Ro real-world study [21], while no severe hypoglycemia episode was reported in another real-world study [20].
A limitation of this study was its non-interventional study design, so potential confounding factors cannot be ruled out. Data collection reflected routine clinical practice rather than mandatory assessments at pre-specified time points, which may have had an impact on the amount of data and its interpretation. Differences in the baseline characteristics among people from different countries and the effect of possible regional differences on clinical outcomes were not determined in this study. In the real world, medication may be retrieved by people later than the date when the prescription was created; however, it is not currently possible to assess the scope and impact of this on the therapeutic dataset. Data on medication costs were not collected, and their impact on treatment intensification was not analyzed in this study. Another key limitation of the study is that the change in the iGlarLixi dose over 6 months was not determined. Considering the real-world setup, the incidence of hypoglycemia may have been underreported in this study.
Conclusion
In a real-world setting, this observational retrospective study demonstrated that iGlarLixi—a fixed-ratio combination of basal insulin glargine 100 U/mL and the short-acting GLP-1 RA lixisenatide—helps people with T2D who need to advance therapy from OADs ± insulin (including premix insulins) to improve glycemic control, while also providing a weight benefit and a low risk of hypoglycemia.
References
Chun J, Strong J, Urquhart S. Insulin initiation and titration in patients with type 2 diabetes. Diabetes Spectr. 2019;32:104–11.
Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427–37.
Paul SK, Klein K, Thorsted BL, et al. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100.
Osataphan S, Chalermchai T, Ngaosuwan K. Clinical inertia causing new or progression of diabetic retinopathy in type 2 diabetes: a retrospective cohort study. J Diabetes. 2017;9:267–74.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45:2753–86.
Rahelić D, Altabas V, Bakula M, et al. Hrvatske smjernice za farmakološko liječenje šećerne bolesti tipa 2 [Croatian guidelines for the pharmacotherapy of type 2 diabetes]. Lijec Vjesn. 2016;138:1–21 (in Croatian).
Rosenstock J, Diamant M, Aroda VR, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof-of-concept randomized trial. Diabetes Care. 2016;39:1579–86.
Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39:2026–35.
Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39:1972–80.
Blonde L, Rosenstock J, Frias J, et al. Durable effects of iGlarLixi up to 52 weeks in type 2 diabetes: the LixiLan-G Extension study. Diabetes Care. 2021;44:774–80.
Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–93.
Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37:2926–33.
Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017;8:101–14.
Rodbard HW, Bode BW, Harris SB, et al. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naive people with type 2 diabetes: the DUAL IV trial. Diabet Med. 2017;34:189–96.
Lingvay I, Perez Manghi F, Garcia-Hernandez P, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315:898–907.
Nuffer W, Guesnier A, Trujillo JM. A review of the new GLP-1 receptor agonist/basal insulin fixed-ratio combination products. Ther Adv Endocrinol Metab. 2018;9:69–79.
Giorgino F, Caruso I, Napoli R. Titratable fixed-ratio combination of insulin glargine plus lixisenatide: a simplified approach to glycemic control in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2020;170: 108478.
European Medical Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. 2012. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus-revision_en.pdf Accessed 3 Apr 2023.
Pintaudi B, Scatena A, Piscitelli G, et al. Clinical profiles and quality of care of subjects with type 2 diabetes according to their cardiovascular risk: an observational, retrospective study. Cardiovasc Diabetol. 2021;20:59.
Kis JT, Nagy G, Kovacs G. Effectiveness of iGlarLixi, a fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide, in people with type 2 diabetes. Diabetes Ther. 2021;12:2517–29.
Bala C, Cerghizan A, Mihai BM, et al. Real-world evidence on the use of a fixed-ratio combination of insulin glargine and lixisenatide (iGlarLixi) in people with suboptimally controlled type 2 diabetes in Romania: a prospective cohort study (STAR.Ro). BMJ Open. 2022;12: e060852.
Sanofi. Suliqua®: EU summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/suliqua-epar-product-information_en.pdf. Accessed 3 Apr 2023.
Acknowledgements
The authors thank the participants of the study and all investigators who contributed to data collection. The authors acknowledged medical writing assistance provided by Deepak Reddy Gade, Ph.D., Sanofi.
Funding
Sponsorship for this study and the journal’s Rapid Service Fee was funded by Sanofi. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Author Contributions
Study concept and design: Danijel Đekić, Mirjana Bojić, Andrej Janež, Sanja Klobučar. Acquisition, analyses, or interpretation: Danijel Đekić, Mirjana Bojić, Andrej Janež, Sanja Klobučar, Iris Grčić Hadžimušović, Tijana Ković, Svetla Mihalevska. Statistical analyses: Danijel Đekić, Mirjana Bojić. Drafting of the manuscript: Danijel Đekić, Mirjana Bojić, Andrej Janež, Sanja Klobučar. Revision of the manuscript and study supervision: Iris Grčić Hadžimušović, Tijana Ković, Svetla Mihalevska.
Disclosures
Danijel Đekic has declared associations (member of the advisory board, lecturer, clinical trial investigator) with the following companies: Eli Lily, Novartis, Novo Nordisk, and Sanofi. Mirjana Bojić has declared associations (lecturer and clinical trial investigator) with the following companies: Novartis, Eli Lily, Novo Nordisk, Boehringer Ingelheim, Takeda, Merck Sharp & Dohme (MSD), Berlin Chemie, Mylan and Sanofi Aventis. Andrej Janež has served as a consultant and is on the Speakers Bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Mediately, Merck Sharp & Dohme (MSD), Novo Nordisk, Medtronic and Sanofi. Sanja Klobučar Majanović has declared associations (lecturer and clinical trial investigator) with Eli Lilly, Merck Sharp & Dohme (MSD), Sanofi Aventis, Novo Nordisk, Abbott, AstraZeneca, Boehringer Ingelheim, Lifescan—Johnson & Johnson, Mylan, Novartis, and Roche. Svetla Mihalevska, Tijana Ković and Iris Grčić hadžimušović were employees of Sanofi at the time of study. Tijana Ković is currently an employee of Optimapharm, and the organization was involved in the performance of the study.
Compliance with Ethics Guidelines
The study was conducted in accordance with the principles laid down by the 18th World Medical Assembly (Declaration of Helsinki, 1964), including all subsequent amendments. It was approved by the regulatory authorities and the independent ethics committees in each of the three countries (Central Ethics Committee [CEC] of the Agency for Medicinal Products and Medical Devices of Croatia, Croatia; The Commission of the Republic of Slovenia for Medical Ethics, Slovenia; Ethics Committee of the Clinical Center of Banja Luka, Bosnia and Herzegovina). Informed consent was obtained from all individual participants prior to inclusion in the study and was signed by those who were on initiated iGlarLixi therapy for at least 6 months. The date of informed consent was considered as the study inclusion visit.
Data Availability
Qualified researchers may request access to patient-level data and related study documents, including the clinical study report, the study protocol with any amendments, a blank case report form, the statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Đekić, D., Bojić, M., Janež, A. et al. Effectiveness and Safety of iGlarLixi in People with Type 2 Diabetes in Adriatic Region Countries: ENSURE-ADR, a Real-World Study. Diabetes Ther 14, 1217–1229 (2023). https://doi.org/10.1007/s13300-023-01407-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01407-3