Abstract
Background
The diagnosis and treatment monitoring of hepatitis C is quite challenging. The screening test, i.e. antibody assay, is unable to detect acute cases, while the gold standard hepatitis C virus (HCV) reverse transcriptase polymerase chain reaction (RTPCR) assay is not feasible in resource-limited countries such as India due to high cost and infrastructure requirement. European Association for the Study of the Liver and World Health Organization have approved a new marker, i.e. HCV core antigen (HCVcAg) assay, as an alternative to molecular assay. In this study, we have evaluated HCVcAg assay for diagnosis and treatment monitoring follow-up in Indian population infected with hepatitis C.
Methods
Blood specimen of 90 clinically suspected cases of acute hepatitis C were tested simultaneously for anti-HCV antibody assay via ELISA (enzyme-linked immunoassay), HCVcAg assay by chemiluminescence immune assay (CLIA) and HCV RTPCR VL (viral load) assay. Thirty-four HCV RTPCR positive patients were further enrolled in treatment monitoring group whose blood samples were tested at the beginning of treatment, two weeks, four weeks and 12 weeks via HCV core Ag assay and HCV RTPCR Viral Load assay.
Results
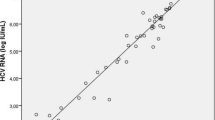
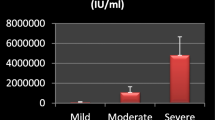
Considering HCV RTPCR as gold standard, diagnostic performance of HCV core Ag assay and anti-HCV antibody assay was evaluated. The sensitivity and specificity of HCV core Ag assay were higher than that of anti-HCV Antibody assay, i.e. 88.3% and 100% vs. 23.3% and 83.3%, respectively. The overall diagnostic accuracy of HCV core Ag assay was 92.20%. Among treatment follow-up group, HCV core Ag levels correlated well with HCV viral load levels, at the beginning of treatment (baseline) till 12 weeks showing highly significant Spearman rank correlation coefficient of > 0.9 with HCV viral load levels.
Conclusions
HCV core Ag assay is a cost-effective, practically feasible substitute of HCV RTPCR viral load assay for diagnosis as well as long duration treatment monitoring of hepatitis C infection in resource-limited settings.
Graphical Abstract


Similar content being viewed by others
Data availability
Not applicable.
References
World Health Organization. Global health sector strategy on viral hepatitis 2016–2021, towards ending viral hepatitis. Geneva: WHO; 2016.
World Health Organization. Guidelines on hepatitis B and C testing. Geneva. Switzerland: World Health Organization; 2017. p. 2017.
Kuo YH, Chang KC, Wang JH, et al. Is hepatitis C virus core antigen an adequate marker for community screening? J Clin Microbiol. 2012;50:1989–93.
Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74.
Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting. 2013. http://www.cdc.gov/hepatitis/hcv/labtesting.htm. Accessed 20 December 2022
Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting. MMWR Morb Mortal 2013;62:362–5. http://www.cdc.gov/mmwr/pdf/wk/mm62e0507a2.pdf. Accessed 20 December 2022
WHO. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. Update version. 2018. https://www.who.int/publications/i/item/9789241550345. Accessed 20 December 2022
Arboledas JCA, Guerrero IP, Rodríguez MJB, et al. Hepatitis C virus core antigen in the management of patients treated with new direct-acting antivirals. Diagn Microbiol Infect Dis. 2017;89:29–34.
Galli C, Julicher P, Plebani M. HCV core antigen comes of age: a new opportunity for the diagnosis of hepatitis C virus infection. Clin Chem Lab Med. 2018;56:880–8.
Benito R, Arribas J, Algarate S, Cebollada R, Gude MJ. Hepatitis C virus core antigen for screening organ donors and recipients. Diagn Microbiol Infect Dis. 2018;91:126–9.
Wang Y, Jie W, Ling J, Yuanshuai H. HCV core antigen plays an important role in the fight against HCV as an alternative to HCV-RNA detection. J Clin Lab Anal. 2021;35:e23755.
Central Bureau of Health Intelligence (2016) Ministry of Health and Family Welfare. National Health Profile. New Delhi, India. https://cbhidghs.mohfw.gov.in/index.
National guidelines for Diagnosis and Management of Viral Hepatitis. Ministry of Health and family welfare, Government of India. National Viral Hepatitis Control Program (NVHCP) National Health Portal Of India. 2018. Accessed 3/4/2023
Alonso R, Pérez-García F, López-Roa P, Alcalá L, Rodeño P, Bouza E. HCV core-antigen assay as an alternative to HCV RNA quantification: a correlation study for the assessment of HCV viremia. Enferm Infecc Microbiol Clin (Engl Ed). 2018;36:175–8.
Rebucci C, Cerino A, Cividini A, Timo L, Furione M, Mondelli MU. Monitoring response to antiviral therapy for patients with chronic hepatitis C virus infection by a core-antigen assay. J Clin Microbiol. 2003;41:3881–4.
Ponnuvel S, Fletcher GJ, Anantharam R, Varughese S, David VG, Abraham P. Clinical utility of hepatitis C virus core antigen (HCVcAg) assay to identify active HCV infection in hemodialysis and renal transplant patients. PLoS One. 2021;16:e0250263.
Solitano V, Plaz Torres MC, Pugliese N, Aghemo A. Management and treatment of hepatitis C: are there still unsolved problems and unique populations? Viruses. 2021;13:1048. https://doi.org/10.3390/v13061048.
Harrison GL, Pryor J, Malani J, et al. Infection frequency of hepatitis C virus and IL28B haplotypes in Papua New Guinea, Fiji, and Kiribati. PLoS One. 2013;8:e66749.
Mateos P. Names, ethnicity and populations; tracing identity in space. Heidelberg: Springer; 2014.
Mateos P. A review of name-based ethnicity classification methods and their potential in population studies. Popul Space Place. 2007;13:243–63.
Kumbhar N, Ramachandran K, Kumar G, Rao Pasupuleti SS, Sharma MK, Gupta E. Utility of hepatitis C virus core antigen testing for diagnosis and treatment monitoring in HCV infection: a study from India. Indian J Med Microbiol. 2021;39(4):462–6.
Kannan A, Biswas L, Kumar A, et al. Improving diagnosis of hepatitis C virus infection using hepatitis C core antigen testing in a resource-poor setting. Rev Soc Bras Med Trop. 2021;10:54.
Reddy AK, Dakshinamurty KV, Lakshmi V. Utility of HCV core antigen ELISA in the screening for hepatitis C virus infection in patients on hemodialysis. Indian J Med Microbiol. 2006;24:55–7.
Medhi S, Potukuchi SK, Polipalli SK, et al. Diagnostic utility of hepatitis C virus core antigen in hemodialysis patients. Clin Biochem. 2008;41:447–52.
Patel J, Sharma P. Design of a novel rapid immunoassay for simultaneous detection of hepatitis C virus core antigen and antibodies. Adv Virol. 2020;165:627–41.
Shah H, Bilodeau M, Burak KW, et al. The management of chronic hepatitis C: 2018 guideline update from the Canadian Association for the Study of the Liver. CMAJ. 2018;190:E677–87.
Adland E, Jesuthasan G, Downs L, et al. Hepatitis virus (HCV) diagnosis and access to treatment in a UK cohort. BMC Infect Dis. 2018;18:461.
Bertisch B, Brezzi M, Negro F, et al. Swiss Hepatitis C Cohort Study. 2020. Very low hepatitis C viral loads in treatment-naive persons: do they compromise hepatitis C virus antigen testing? Clin Infect Dis 70:653–9. https://doi.org/10.1093/cid/ciz270
Acknowledgements
We would like to acknowledge Dr Vikram Singh, Additional Professor, Department of Medicine, Dr RMLIMS, Lucknow, for his administrative help in conducting the study.
Author information
Authors and Affiliations
Contributions
Jaya Garg: conceptualized and did overall supervision of the study. Prashant Verma: clinical analysis of admitted patients. Mridu Singh: helped in clinical evaluation and follow-up of enrolled patients. Anupam Das: laboratory testing and analysis. Anurag Pathak: compiled the data and calculated the statistical results of the study. Jyotsna Agarwal: helped in treatment monitoring of patients. I/we affirm that the submission represents original work that has not been published previously and is not currently being considered or submitted to another journal, until a decision has been made. Also, we confirm that each author has seen and approved the contents of the submitted manuscript. This manuscript has the permission from the institutional IRB.
Corresponding author
Ethics declarations
Competing interests
JG, PV, MS, AD, AP and JA declare no competing interests.
Ethics statement
The study was performed conforming to the Helsinki Declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Ethical approval
Ethical approval has been granted by Institute ethical committee(IEC-No 26/19).
Consent to participate
All subjects enrolled in the study have given written consent to participate in study.
Consent for publication
All authors have seen and approved the contents of the submitted manuscript and given the consent for publication.
Human ethics
Not applicable.
Support in form of grants
Intramural project grant.
Disclaimer
The authors are solely responsible for the data and the content of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garg, J., Verma, P., Singh, M. et al. Hepatitis C virus core antigen: A diagnostic and treatment monitoring marker of hepatitis C virus in Indian population. Indian J Gastroenterol (2024). https://doi.org/10.1007/s12664-024-01549-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12664-024-01549-7