Abstract
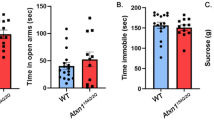
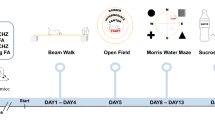
Spinocerebellar ataxia type 2 (SCA2) is a hereditary disorder, caused by an expansion of polyglutamine in the ataxin-2 protein. Although the mutant protein is expressed throughout all the cell and organ types, the cerebellum is primarily affected. The disease progression is mainly accompanied by a decline in motor functions. However, the disturbances in cognitive abilities and low mental state have also been reported in patients. Recent evidence suggests that the cerebellar functionality expands beyond the motor control. Thus, the cerebellum turned out to be involved into the language, verbal working, and spatial memory; executive functions such as working memory, planning, organizing, and strategy formation; and emotional processing. Here, we used the transgenic SCA2-58Q mice to evaluate their anxiety, cognitive functions, and mood alterations. The expression of the mutant ataxin-2 specifically in the cerebellar Purkinje cells (PCs) in SCA2-58Q mice allowed us to study the direct involvement of the cerebellum into the cognitive and affective control. We determined that SCA2-58Q mice exhibit anxiolytic behavior, decline in spatial memory, and a depressive-like state. Our results support the idea of cerebellar involvement in cognitive control and the handling of emotions.









Similar content being viewed by others
Data Availability
Materials availability—this study did not generate new unique reagents. Data are available upon request.
References
Matilla-Duenas A, Sanchez I, Corral-Juan M, Davalos A, Alvarez R, Latorre P. Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum. 2009.
Carlson KM, Andresen JM, Orr HT. Emerging pathogenic pathways in the spinocerebellar ataxias. Curr Opin Genet Dev. 2009;19(3):247–53.
Egorova PA, Bezprozvanny IB. Inositol 1,4,5-trisphosphate receptors and neurodegenerative disorders. FEBS J. 2018;285(19):3547–65.
Egorova PA, Bezprozvanny IB. Molecular mechanisms and therapeutics for spinocerebellar ataxia type 2. Neurotherapeutics. 2019;16(4):1050–73.
Hekman KE, Gomez CM. The autosomal dominant spinocerebellar ataxias: emerging mechanistic themes suggest pervasive Purkinje cell vulnerability. J Neurol Neurosurg Psychiatry. 2015;86(5):554–61.
Pirker W, Back C, Gerschlager W, Laccone F, Alesch F. Chronic thalamic stimulation in a patient with spinocerebellar ataxia type 2. Mov Disord. 2003;18(2):222–5.
Wang Z. Experimental and clinical strategies for treating spinocerebellar ataxia type 3. Neuroscience. 2018;371:138–54.
Wagner JL, O’Connor DM, Donsante A, Boulis NM. Gene, stem cell, and alternative therapies for SCA 1. Front Mol Neurosci. 2016;9:67.
Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75.
De Zeeuw CI, Lisberger SG, Raymond JL. Diversity and dynamism in the cerebellum. Nat Neurosci. 2021;24(2):160–7.
Jacobi H, Faber J, Timmann D, Klockgether T. Update cerebellum and cognition. J Neurol. 2021;268(10):3921–5.
Stoodley CJ, Schmahmann JD. The cerebellum and language: evidence from patients with cerebellar degeneration. Brain Lang. 2009;110(3):149–53.
Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501.
Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 2010;23(1–2):65–79.
Larry N, Yarkoni M, Lixenberg A, Joshua M. Cerebellar climbing fibers encode expected reward size. Elife. 2019;8.
Heffley W, Hull C. Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum. Elife. 2019;8.
Kostadinov D, Beau M, Blanco-Pozo M, Hausser M. Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat Neurosci. 2019;22(6):950–62.
Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science. 2019;363(6424).
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79.
D’Mello AM, Crocetti D, Mostofsky SH, Stoodley CJ. Cerebellar gray matter and lobular volumes correlate with core autism symptoms. Neuroimage Clin. 2015;7:631–9.
Chen YL, Tu PC, Lee YC, Chen YS, Li CT, Su TP. Resting-state fMRI mapping of cerebellar functional dysconnections involving multiple large-scale networks in patients with schizophrenia. Schizophr Res. 2013;149(1–3):26–34.
Liang MJ, Zhou Q, Yang KR, Yang XL, Fang J, Chen WL, Huang Z. Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting-state FMRI. PLoS One. 2013;8(12):e79999.
Bledsoe JC, Semrud-Clikeman M, Pliszka SR. Neuroanatomical and neuropsychological correlates of the cerebellum in children with attention-deficit/hyperactivity disorder–combined type. J Am Acad Child Adolesc Psychiatry. 2011;50(6):593–601.
Liu L, Zeng LL, Li Y, Ma Q, Li B, Shen H, Hu D. Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PLoS One. 2012;7(6):e39516.
Le Pira F, Zappala G, Saponara R, Domina E, Restivo D, Reggio E, Nicoletti A, Giuffrida S. Cognitive findings in spinocerebellar ataxia type 2: relationship to genetic and clinical variables. J Neurol Sci. 2002;201(1–2):53–7.
Burk K, Globas C, Bosch S, Klockgether T, Zuhlke C, Daum I, Dichgans J. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol. 2003;250(2):207–11.
Fancellu R, Paridi D, Tomasello C, Panzeri M, Castaldo A, Genitrini S, Soliveri P, Girotti F. Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J Neurol. 2013;260(12):3134–43.
Gigante AF, Lelli G, Romano R, Pellicciari R, Di Candia A, Mancino PV, Pau M, Fiore P, Defazio G. The relationships between ataxia and cognition in spinocerebellar ataxia type 2. Cerebellum. 2020;19(1):40–7.
Moriarty A, Cook A, Hunt H, Adams ME, Cipolotti L, Giunti P. A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7. Orphanet J Rare Dis. 2016;11(1):82.
Paneque HM, Reynaldo AR, Velazquez PL, Santos FN, Miranda HE, Real PN, Garcia ER, Hechavarria PR. Type 2 spinocerebellar ataxia: an experience in psychological rehabilitation. Rev Neurol. 2001;33(11):1001–5.
Olivito G, Lupo M, Iacobacci C, Clausi S, Romano S, Masciullo M, Molinari M, Cercignani M, Bozzali M, Leggio M. Microstructural MRI basis of the cognitive functions in patients with spinocerebellar ataxia type 2. Neuroscience. 2017;366:44–53.
Olivito G, Lupo M, Iacobacci C, Clausi S, Romano S, Masciullo M, Molinari M, Cercignani M, Bozzali M, Leggio M. Structural cerebellar correlates of cognitive functions in spinocerebellar ataxia type 2. J Neurol. 2018;265(3):597–606.
Huynh DP, Figueroa K, Hoang N, Pulst SM. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet. 2000;26(1):44–50.
Egorova PA, Zakharova OA, Vlasova OL, Bezprozvanny IB. In vivo analysis of cerebellar Purkinje cell activity in SCA2 transgenic mouse model. J Neurophysiol. 2016;115(6):2840–51.
Egorova PA, Gavrilova AV, Bezprozvanny IB. In vivo analysis of the climbing fiber-Purkinje cell circuit in SCA2-58Q transgenic mouse model. Cerebellum. 2018;17(5):590–600.
Egorova PA, Gavrilova AV, Bezprozvanny IB. In vivo analysis of the spontaneous firing of cerebellar Purkinje cells in awake transgenic mice that model spinocerebellar ataxia type 2. Cell Calcium. 2021;93:102319.
Kasumu AW, Hougaard C, Rode F, Jacobsen TA, Sabatier JM, Eriksen BL, Strobaek D, Liang X, Egorova P, Vorontsova D, Christophersen P, Ronn LC, Bezprozvanny I. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem Biol. 2012;19(10):1340–53.
Kasumu AW, Liang X, Egorova P, Vorontsova D, Bezprozvanny I. Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci. 2012;32(37):12786–96.
Bohne P, Mourabit DB, Josten M, Mark MD. Cognitive deficits in episodic ataxia type 2 mouse models. Hum Mol Genet. 2021;30(19):1811–32.
Lopatina O, Yoshihara T, Nishimura T, Zhong J, Akther S, Fakhrul AA, Liang M, Higashida C, Sumi K, Furuhara K, Inahata Y, Huang JJ, Koizumi K, Yokoyama S, Tsuji T, Petugina Y, Sumarokov A, Salmina AB, Hashida K, Kitao Y, Hori O, Asano M, Kitamura Y, Kozaka T, Shiba K, Zhong F, Xie MJ, Sato M, Ishihara K, Higashida H. Anxiety- and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for Parkinson’s disease. Front Behav Neurosci. 2014;8:133.
Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13(2):167–70.
Lueptow LM. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J Vis Exp. 2017 ;(126).
Asher M, Rosa JG, Cvetanovic M. Mood alterations in mouse models of Spinocerebellar Ataxia type 1. Sci Rep. 2021;11(1):713.
Huynh DP, Maalouf M, Silva AJ, Schweizer FE, Pulst SM. Dissociated fear and spatial learning in mice with deficiency of ataxin-2. PLoS One. 2009;4(7):e6235.
Nakajima R, Takao K, Hattori S, Shoji H, Komiyama NH, Grant SGN, Miyakawa T. Comprehensive behavioral analysis of heterozygous Syngap1 knockout mice. Neuropsychopharmacol Rep. 2019;39(3):223–37.
Stezin A, Bhardwaj S, Hegde S, Jain S, Bharath RD, Saini J, Pal PK. Cognitive impairment and its neuroimaging correlates in spinocerebellar ataxia 2. Parkinsonism Relat Disord. 2021;85:78–83.
Mastammanavar VS, Kamble N, Yadav R, Netravathi M, Jain S, Kumar K, Pal PK. Non-motor symptoms in patients with autosomal dominant spinocerebellar ataxia. Acta Neurol Scand. 2020;142(4):368–76.
Phillips JR, Hewedi DH, Eissa AM, Moustafa AA. The cerebellum and psychiatric disorders. Front Public Health. 2015;3:66.
Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288(14):1740–8.
Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, Sharp WS, Giedd JN, Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164(4):647–55.
Duan K, Jiang W, Rootes-Murdy K, Schoenmacker GH, Arias-Vasquez A, Buitelaar JK, Hoogman M, Oosterlaan J, Hoekstra PJ, Heslenfeld DJ, Hartman CA, Calhoun VD, Turner JA, Liu J. Gray matter networks associated with attention and working memory deficit in ADHD across adolescence and adulthood. Transl Psychiatry. 2021;11(1):184.
Kraskovskaya NA, Erofeev AI, Grishina ED, et al. Development of hippocampus-associated cognitive dysfunction in Huntington’s disease mouse model. J Evol Biochem Phys. 2021;57:1449–60.
Asher M, Rosa JG, Rainwater O, Duvick L, Bennyworth M, Lai RY, Kuo SH, Cvetanovic M. Cerebellar contribution to the cognitive alterations in SCA1: evidence from mouse models. Hum Mol Genet. 2020;29(1):117–31.
Valis M, Masopust J, Bazant J, Rihova Z, Kalnicka D, Urban A, Zumrova A, Hort J. Cognitive changes in spinocerebellar ataxia type 2. Neuro Endocrinol Lett. 2011;32(3):354–9.
Hult LS, Nilsson N, Soylu R, Kirik D, Petersen A. Hypothalamic expression of mutant huntingtin contributes to the development of depressive-like behavior in the BAC transgenic mouse model of Huntington’s disease. Hum Mol Genet. 2013;22(17):3485–97.
Minichino A, Bersani FS, Trabucchi G, Albano G, Primavera M, Delle CR, Biondi M. The role of cerebellum in unipolar and bipolar depression: a review of the main neurobiological findings. Riv Psichiatr. 2014;49(3):124–31.
Mills NP, Delbello MP, Adler CM, Strakowski SM. MRI analysis of cerebellar vermal abnormalities in bipolar disorder. Am J Psychiatry. 2005;162(8):1530–2.
Peng J, Liu J, Nie B, Li Y, Shan B, Wang G, Li K. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur J Radiol. 2011;80(2):395–9.
Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, Pulst SM, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29(29):9148–62.
Acknowledgements
We are grateful to members of the Laboratory of Molecular Neurodegeneration for advice and suggestions, to John E. Roberts III for technical assistance and encouragement, and to Anastasia V. Bolshakova for administrative assistance. IB is a holder of the Carl J. and Hortense M. Thomsen Chair in Alzheimer’s Disease Research.
Funding
This work was supported by the Russian Science Foundation Grant 22–75-10030 (PE) and by the National Institutes of Health grant R33NS101182 (IB). The financial support was divided in the following way: research work related to Figs. 1, 2, 3, 4, 5, 6, 7, 8, and 9 was supported by the Russian Science Foundation Grant 22–75-10030, and the publication fee was supported by the NIH grant.
Author information
Authors and Affiliations
Contributions
Conceptualization: P.E and I.B. Data curation: K.M. and P.E. Funding acquisition: P.E. and I.B. Formal Analysis: K.M. and P.E. Investigation: K.M. and P.E Methodology: K.M. and P.E. Project administration: P.E. and I.B. Resources: P.E. and I.B. Software: K.M. and P.E. Supervision: I.B. Validation: K.M. and P.E. Visualization: K.M. and P.E. Writing—original draft: K.M.and P.E. Writing—review and editing: P.E. and I.B.
Corresponding authors
Ethics declarations
Ethics Approval
All experimental protocols were approved by the Bioethics Committee of the Peter the Great St. Petersburg Polytechnic University at St. Petersburg, Russia, and followed the principles of the European convention (Strasbourg, 1986) and the Declaration of International medical association about the humane treatment of animals (Helsinki, 1996). All methods were carried out in accordance with relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marinina, K.S., Bezprozvanny, I.B. & Egorova, P.A. Cognitive Decline and Mood Alterations in the Mouse Model of Spinocerebellar Ataxia Type 2. Cerebellum 23, 145–161 (2024). https://doi.org/10.1007/s12311-023-01520-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-023-01520-w