Abstract
Not much is known about the effects of sodium-glucose cotransporter 2 inhibitors (SGLT2i) on echocardiographic parameters of left ventricular (LV) systolic function in patients with heart failure and reduced ejection fraction (HFrEF).
We prospectively included 59 outpatients with HFrEF: 41 patients received SGLT2i with OMT (SGLT2i+ group), whereas eighteen patients received OMT without SGLT2i (SGLT2i− group). Myocardial work index (MWI), 3D ejection fraction (3D LVEF), and global longitudinal strain (GLS) were measured at baseline and after 3 months following treatment. At 3-month follow-up, the SGLT2i+ group showed significantly greater improvement in MWI than the SGLT2i− group. In both groups, there was a significant improvement in 3D LVEF and LV GLS, circulating NT-proBNP levels, and NYHA functional class, with significantly greater improvement in the SGLT2i+ group.
In conclusion, the addition of SGLT2i to fully optimized background medical therapy resulted in a greater improvement of LV systolic function among outpatients with HFrEF.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key Messages
What Is Already Known on This Topic?
-
It is known that SGLT2 inhibition in heart failure with reduced ejection fraction (HFrEF) promotes reverse cardiac remodeling and is associated with better clinical outcomes.
-
SGLT2 inhibition has been associated with reduced left ventricular (LV) and left atrial volumes in HFrEF; however, its relationship with contemporary echocardiographic parameters of global LV systolic function, including global longitudinal strain and myocardial work have not been fully investigated.
What Does This Study Adds?
-
In outpatients with HFrEF, addition of dapagliflozin or empagliflozin to optimal background medical therapy (OMT) was associated with a significant improvement in global work index and global work efficiency compared to OMT without SGLT2 inhibitor.
-
Outpatients with HFrEF receiving SGLT2 inhibitor also had a more meaningful improvement in 3D left ventricular ejection fraction and global longitudinal strain compared to those receiving OMT without SGLT2 inhibitor, after the 3-month follow-up.
-
Similarly, addition of SGLT2 inhibitor to sacubitril/valsartan, beta-blocker, and mineralocorticoid receptor antagonist was associated with a greater reduction in circulating natriuretic peptides and NYHA functional class.
How Might This Study Affect Research, Practice, or Policy?
-
This study shows that adding vs. omitting SGLT2 inhibitor to OMT in outpatients with HFrEF was associated with a significantly greater improvement in LV systolic function accompanied with a more robust reduction in markers of neurohumoral burden and NYHA class during the 3-month follow-up.
Introduction
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have an established place in the therapy of heart failure with reduced ejection fraction (HFrEF), as they have been shown to significantly reduce all-cause mortality, cardiovascular mortality, and hospitalizations in HFrEF patients, regardless of the presence of diabetes mellitus [1, 2]. The growing evidence of the effect of SGLT2i on cardiac remodeling through cellular, molecular, vascular, interstitial, and electrical effects justifies its role as fundamental HFrEF therapy [3,4,5]. Measurement of the effect of SGLT2i therapy on left ventricular systolic function (LV) has previously been assessed by changes in heart size and volume and changes in ejection fraction [6]. In current clinical practice, the assessment of LV systolic function is still mainly based on the measurement of LV ejection fraction (LVEF), which remains the gold standard despite some notable limitations [7].
In the last decade, LV global longitudinal strain (GLS) has emerged as a more accurate predictor of poor outcomes, revealing subtle abnormalities and preclinical LV systolic dysfunction [8]. The disadvantage of GLS and LVEF measurements is their dependence on pressure and volume loading [9]. Global myocardial work index (GWI) is a new load-independent echocardiographic parameter derived from pressure-strain loops. The GWI came into the limelight after Russell and colleagues demonstrated a good correlation of the invasively measured pressure-volume loop with the noninvasive pressure-strain loop (PSL) as an equivalent for myocardial performance [10, 11]. Of interest, studies have shown that GWI reflects regional glucose metabolism of LV compared to 18-fluorodeoxyglucose-positron emission tomography (PET) [10]. Taken together, a growing body of data suggests that GWI measurement may have additional value as a predictor of outcomes and response to pharmacological treatment in many cardiovascular diseases such as HF [12].
The present study prospectively investigated the effect of 3-month treatment with SGLT2i added to optimal medical therapy (OMT) on echocardiographic myocardial work indices, compared to the OMT control group. SGLT2i were added to maximally tolerated doses of OMT including sacubitril-valsartan (ARNI), mineralocorticoid antagonist (MRA), and beta-blocker (BB). We also investigated the change in plasma levels of N-terminal-pro-b-type natriuretic peptide (NT-proBNP) and functional status (as assessed by the New York Heart Association (NYHA) scale), after 3 months of addition of SGLT2i vs continuing OMT alone.
Methods
This study was designed as a single-center, prospective, single-blinded study in which the echocardiographic expert (sonographer) was blinded regarding treatment assignment (SGLT2i administered vs. not administered as an adjunct to optimized background medical therapy). The study was conducted at the Cardiovascular Diseases Department, University Hospital of Split, Croatia, from March 2021 to April 2022. The study was conducted in full compliance with the principles of the Declaration of Helsinki from 2013 and with the approval of the Ethics Committee of the University Hospital of Split, filed under number 2181-147/01/06/M.S.-20-02. All participants read and signed the informed consent.
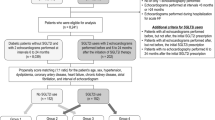
We consecutively enrolled outpatients with HFrEF on optimized medical therapy defined by the guidelines of the European Society of Cardiology (ESC) that were in use at the time when the study was initiated (2016 edition) [13]. The study included patients with a diagnosis of HFrEF according to ESC guidelines for diagnosis and management of acute and chronic heart failure [13] aged 18 or older with LVEF <40%. At the time of inclusion, patients already had to be on guideline-recommended OMT including sacubitril-valsartan (ARNI), beta-blocker (BB), and mineralocorticoid receptor antagonists (MRA) on the highest tolerable daily doses, in addition to other symptomatic therapy such as loop diuretics. Furthermore, only patients with functional symptom severity class II and III on the NYHA scale with concomitant NT-proBNP value >125 pg/mL were included in the study. We excluded patients that had symptomatic hypotension (systolic blood pressure < 95 mmHg), impaired renal function (eGFR < 30 ml/min/1.73 m2 calculated by the CKD-EPI formula), and potassium serum levels > 5.2 mmol/L, liver dysfunction (defined as hepatic parameters such as ALT, AST, and/or ALP elevated ≥3 times above the upper 99th reference percentile), biliary cirrhosis and cholestasis, active malignant disease (irrelevant of the stage and type of malignancy), current use of hormonal therapy, chemotherapy or immunotherapy, presence of the artificial (mechanical or biological) heart valve, severe aortic stenosis, acute coronary syndrome within the last 3 months, percutaneous coronary intervention or acute cerebrovascular incident within 3 months prior to the date of enrollment, pregnancy, or breastfeeding. Furthermore, due to potential interaction with left ventricular structure and function, patients with diabetes mellitus treated with DPP4 inhibitors and GLP receptor agonists were excluded from the study. Finally, patients that were not able to provide written informed consent or declined to participate in the study were not enrolled. Similarly, from the primary analysis, we excluded all patients that withdrew from the study due to any reason or that suffered any major medical condition not related to primary disease but with the potential to affect cardiovascular outcomes or lead to death before the study completion.
From March 2021 until September 2021, 47 patients were randomized into two groups using a random number generator: (a) SGLT2i+ group (either empagliflozin or dapagliflozin 10 mg once daily added to the current OMT, N = 26); and (b) SGLT2i− group (a group of patients treated with the continuation of identical OMT but without the addition of SGLT2 inhibitor, N = 21). While the study was ongoing in September 2021, new ESC guidance was published recommending the use of SGLT2 inhibitors as a foundational drug in HFrEF management (class recommendation IA) [5]. Due to this fact, all HFrEF patients enrolled in the study after September 2021 (N = 16) were prescribed an SGLT2i resulting in a 2:1 ratio between patients with added SGLT2 vs. no SGLT2 inhibitor, 42 vs. 21 patients, respectively. There was no crossover between the groups, nor were there any changes in the daily dosage of their fundamental therapy (the same starting dose of OMT was given during the 3-month follow-up period). Patients from the group without SGLT2i after follow-up were assigned to SGLT2i according to the newest recommendation but were not followed further.
All patients underwent a comprehensive echocardiographic examination, physical examination, medical interview, and biochemical laboratory testing at baseline and repeated at the 3-month follow-up examination (3mFU). All echocardiographic measurements were performed by the same certified echocardiography consultant, blinded to the participant's treatment allocation, and, at the end of the study, validated by another cardiology consultant to determine the possibility of measurement error. A detailed description of the procedures performed on each participating patient is provided in the Appendix 1.
An echocardiographic examination included measurements of 2D speckle tracking echocardiography with LV global longitudinal strain (GLS, −%) assessment, global myocardial work analysis encompassing global work index (GWI, mmHg%), global work efficiency (GWE, %), global constructive work (GCW, mmHg%) and global wasted work (GWW, mmHg%), and three-dimensional measurements of volumes (mL) and LVEF (%).
The primary outcome was defined as the change in echocardiographic parameters of myocardial work and other parameters of myocardial function at 3-month follow-up compared to baseline in each group and whole cohort. Secondary outcomes included (a) mean changes in echocardiographic parameters of myocardial work and other parameters of myocardial function from baseline to 3-month follow-up compared between two groups; (b) subanalysis of predefined endpoints among patients with added SGLT2i stratified by sex, diabetes mellitus, and etiology of cardiomyopathy (dilated vs. ischemic cardiomyopathy).
Statistical Analysis
All data analyses were performed with SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA) and Prism, version 9.0.1. (GraphPad, La Jolla, CA, USA). Continuous data were presented as mean ± standard deviation (SD) whereas categorical variables were shown as whole numbers (N) and percentages (%). The normality of data distribution was examined with the Kolmogorov-Smirnov test. The t-test for independent samples was utilized to measure potential differences between two groups of interest (SGLT2i users vs. SGLT2i non-users and other subgroups, as appropriate). The echocardiographic variables of interest, NYHA functional class, and circulating NT-proBNP levels were specifically examined in the “before-after” fashion in which initial values (at baseline) were pairwise compared to values obtained at 3-month follow-up by using paired t-test for within the group analyses. For the “before-after” comparison analysis between two groups of interest (SGLT2i+ group vs. SGLT2i− group), we used repeated measures ANOVA analysis. The mean change (Δ, delta) represented the average numerical change from baseline to follow-up at all instances. The Chi-square (χ2) test was used to examine differences between groups of interest with respect to categorical variables. In all cases, two-sided significant (p) values were reported, and results that reached p < 0.05 were considered statistically significant.
Results
A total of 63 consecutive HFrEF patients were enrolled in the study with the intention to treat (ITT). During the planned follow-up period of 3 months (3mFU), four patients (6.3% of the total intention-to-treat number) were excluded from the study. The reasons were as follows: (a) one patient from the SGLT2i+ group withdrew consent to participate due to relocation to another state; (b) two patients from the SGLT2i− group suffered from a severe form of COVID-19 infection requiring machine respiratory support and consequently had impaired cardiac function; (c) one patient from the SGLT2i− group died after 5 weeks of follow-up due to sudden cardiac death. A total of 59 patients (41 patients in the SGLT2i+ group and 18 patients in the SGLT2i− group) completed the study per the protocol, and their data were analyzed. Among these patients, none had infections or unplanned hospitalizations during the three months follow-up.
Baseline characteristics of patients in both groups (SGLT2i+ vs.SGLT2i−) did not differ significantly with respect to age, sex, NYHA status, arterial blood pressure, etiology of cardiomyopathy, renal function, and circulating NT -proBNP levels (Table 1). All patients in each group were taking ARNI, BBs, and MRAs at baseline, and these drugs were titrated to the maximum tolerated daily dose. Both groups were similar with respect to the mean daily dose of these baseline medications. The SGLT2i+ group had a significantly higher prevalence of diabetes mellitus at baseline than the SGLT2i nonusers group (39 vs. 11%, respectively). The burden of other comorbidities (hypertension, hyperlipidemia, atrial fibrillation) was similar between the groups.
Patients took sacubitril/valsartan for 3–11 months and BB and MRA for 12–22 months. All included patients received bisoprolol as BB, and those patients receiving loop diuretics all received furosemide.
Myocardial Work Parameters
Significant improvement in all myocardial work parameters from baseline to follow-up was observed in the entire cohort (Fig. 1), with significantly greater improvement in the group of patients who received SGLT2i. The group that did not receive SGLT2i did not show significant before-after improvement in three of four MW indices (GCW p = 0.098, GWW p = 0.202, GWE p = 0.194), except for GWI (p = 0.043), while this was achieved in the group receiving SGLT2i for all indices (GCW p < 0.001, GWW p =0.048, GWE p < 0.001, GWI p < 0.001) (Fig. 1). Notably, in the SGLT2i+ group GWI increased by 499 ± 504 mm Hg%, while in the SGLT2i− group, the increase was 224 ± 315 mm Hg%, p = 0.035 (Fig. 1a, Table 2). Similarly, GWE increased by 8.7 ± 10.1 % in the SGLT2i+ group and by 2.9 ± 9.3 % in the SGLT2i− group, p = 0.042 (Fig. 1b, Table 2). While the improvement in the indices of GCW and GWW was numerically more in the SGLT2i+ group than in the SGLT2i− group, these results did not reach statistical significance (p = 0.084 and p =0.894 respectively) (Fig. 1c, d, respectively; Table 2).
Left images: changes from baseline to follow-up in the whole cohort, group without SGLT2 inhibitors, and group with SGLT2 inihibitor. Right images: comparison of mean changes between group with and without SGLT2 inhibitor. a differences in global myocardial work index (GWI); b differences in global work efficiency (GWE); c differences in global constructive work (GCW); d differences in global wasted work (GWW); *t-test of independent samples; all results were significant at two-tailed p-value < 0.05
3D Left Ventricular Ejection Fraction and LV Global Longitudinal Strain
The entire cohort achieved statistically significant improvement from baseline to follow-up in 3D LVEF and LV GLS parameters (p < 0.001 and p < 0.001, respectively) (Fig. 2). Both groups achieved statistically significant improvement in 3D LVEF from baseline to follow-up, but a significantly higher magnitude of improvement was observed in the SGLT2i+ group (12.2 ± 8.9 %, p < 0.001), compared to the SGLT2i− group (5.1 ± 9.9 %, p = 0.043), between groups p = 0.009. The same was observed in the results of LVGLS. In the SGLT2i+ group, the improvement of LV GLS was 3.9 ± 3.6 % (p < 0.001) compared to 1.6 ± 2.7 % (p = 0.018) in the SGLT2i− group, between groups p = 0.023 (Fig. 2a, b, Table 2).
Left images: changes from baseline to follow-up in the whole cohort, group without SGLT2 inhibitors, and group with SGLT2 inihibitor. Right images: comparison of mean changes between group with and without SGLT2 inhibitor. a differences in 3D left ventricular ejection fraction (LVEF); b differences in global longitudinal strain (GLS) of left ventricle; c differences in circulating N-terminal-pro-brain natriuretic peptide (NT-proBNP) levels; d differences in New York Heart Association (NYHA) functional status ; *t-test of independent samples; all results were significant at two-tailed p-value < 0.05
NT-proBNP and NYHA Functional Class
Both groups showed a statistically significant decrease in circulating NT-proBNP levels and improvement in NYHA-class (p < 0.001 and p = 0.003, respectively) (Fig. 2c, d). The decrease in circulating NT-proBNP levels, from baseline to follow-up, was significant in the SGLT2i+ group (−3790 ± 5665 pg/mL, p < 0.001), whereas this change was not significant in the SGLT2i− group (−701 ± 3438 pg/mL, p = 0.061) (Fig. 2c, Table 2.). When comparing the mean change in NT-proBNP levels between groups, the SGLT2i+ group showed a significantly greater decrease in NT-proBNP levels compared to the SGLT2i− group, p = 0.003 (Table 2). Functional syndrome capacity as assessed by the NYHA scale, from baseline to follow-up, improved significantly in the SGLT2i+ group, whereas this was not the case in the SGLT2i− group (Fig. 2d). In the SGLT2+ group, there was a mean decrease in functional burden assessed by the NYHA scale by 0.40 ± 0.58 points (p < 0.001), whereas functional burden remained nearly the same in the SGLT2i− group (NYHA function increase by 0.06 ± 0.54 points, p = 0.668), as shown in Table 2.
The Proportion of Patients Who Achieved Significant Improvement in NT-proBNP Reduction and GWI Increases at Follow-Up
As shown in Fig. 3, a significantly greater proportion of patients in the SGLT2i+ group achieved NT-proBNP levels <1000 pg/mL at follow-up, compared to the SGLT2i− group (68.3 vs. 39.0%, respectively, p = 0.034). Similarly, the GWI greater than 750 mm Hg% at follow-up was achieved more frequently by patients treated with SGLT2i compared to patients not treated with SGLT2i (80.5 vs. 50.0%, respectively p = 0.017).
The left side of the image shows the proportion of the patients from the group without SGLT2i (top image) and the group with addition of SGLT2i (bottom image) in terms of reaching the cut-off value of NT-proBNP levels below 1000 pg/mL; the right side of the image shows the proportion of patients from the group without SGLT2i (top image) and the group with addition of SGLT2i (bottom image) in terms of reaching the cut-off value of GWI greater than 750 mm Hg%
Subanalysis in Relation to Sex, Diabetes Status, and Cardiomyopathy Type in Patients Receiving an SGLT2 Inhibitor
Patients treated with SGLT2i did not differ in predefined endpoints when stratified by sex, diabetes mellitus, and etiology of cardiomyopathy (dilated non-ischemic vs. ischemic cardiomyopathy), as shown in Table 3, except for the greater reduction in GWW values in patients with dilated vs. ischemic cardiomyopathy (p = 0.023).
Discussion
In our prospective follow-up study, we observed significant improvement in GWI, GWE, GLS, and 3D-LVEF in HFrEF outpatients receiving SGLT2i (empagliflozin or dapagliflozin) compared with well-matched HFrEF outpatients not receiving SGLT2i, with concomitant reduction in NT-proBNP levels and improvement in functional status.
Our study provides new data on the effects of SGLT2 inhibitors on advanced echocardiographic parameters of left ventricular systolic function in ambulatory patients with chronic HFrEF, which have been understudied in previous studies. To date, several studies have examined the effects of SGLT2i treatment on the LV function [14]. The only quantification of the effect of SGLT2i on echocardiographic myocardial work parameters was shown by Ikonomidis et al. who studied the effect of insulin, GLP-1RA, SGLT2i, and their combination in patients with type 2 diabetes mellitus (T2DM) independent of baseline LVEF values or HFrEF therapy [15]. Their study showed a greater increase in GWI among patients using GLP-1RA or a combination of GLP-1RA and SGLT2i compared with insulin-only users [15]. In addition, myocardial work indices were used to evaluate the therapeutic effect when sacubitril/valsartan was added to BB and MRA during 6 months of therapy and showed an improvement in GCW and GWE [16, 17]. Therefore, to the best of our knowledge, the present study is the first to investigate and quantify the effects of adding SGLT2i to maximally optimized medical therapy, consisting of sacubitril/valsartan, MRA, and BB, on myocardial work indices in the population of ambulatory HFrEF patients.
An important role of GWI was first demonstrated by Wang et al. in their retrospective analysis, which showed that HFrEF patients with a GWI of <750 mm Hg% had significantly more adverse outcomes such as all-cause death and HF hospitalization, compared to patients with higher values [18]. In our study, a significantly higher proportion of patients in the SGLT2i+ group had recovery of GWI above the cut-off value of 750 mm Hg% compared with the group that did not use SGLT2i. Furthermore, our study showed that both treatment groups had significant improvement from baseline to follow-up in terms of LV GLS, but the recovery in LV GLS was more pronounced in SGLT2 users. Lee et al. failed to demonstrate a difference in GLS with empagliflozin compared with placebo [19], in contrast to the results of Gamaza-Chulian et al. who demonstrated a significant improvement in GLS associated with the use of SGLT2i in patients with T2DM [20].
In the present study, 3D-estimated LVEF improved significantly in both treatment groups, but in agreement with previous results, a significantly greater improvement was achieved in SGLT2 inhibitor users compared with nonusers. A post hoc analysis of the EMPIRE-HF study, which focused on left ventricular remodeling in HFrEF patients treated with empagliflozin versus placebo after 12 weeks of therapy, showed a significant reduction in LV and left atrial volume without a relevant change in LVEF. It should be noted, however, that only 33% of patients in this study were taking ARNI, whereas approximately 60% were taking MRA, which contrasts with our study in which all patients were taking maximum tolerated daily doses of background therapy of ARNI, MRA, and BB [21]. The estimation of LVEF by TTE showed no significant improvement associated with the addition of SGLT2i so far [14]. Estimation of LVEF using the Simpson biplane method is dependent on the image plane and other geometric assumptions [22], whereas 3D measurement of LVEF offers better reproducibility and less inter- and intra-observer variability. In addition, 3D LVEF appears to be a better predictor of adverse events than 2D LVEF and has a strong correlation with LVEF measured by CMR [22, 23].
From the biochemical point of view of neurohumoral stress on LV, our study showed that the addition of SGLT2i to HFrEF background therapy was associated with a significantly greater reduction in circulating levels of NT-proBNP at 3 months compared with no addition. Previous studies have shown that NT-proBNP levels < 1000 pg/mL achieved at follow-up may serve as a robust predictor of lower mortality and future hospitalization rates in HFrEF patients [24]. Consistent with these observations, in our study, a greater proportion of patients in the SGLT2i+ group had their NT-proBNP levels reduced to less than 1000 pg/mL compared with the SGLT2i− group. The study EMPIRE-HF, which enrolled low-risk HFrEF patients with mild symptoms treated with empagliflozin for 12 weeks, showed neither a significant reduction in serum levels of NT-proBNP nor an improvement in daily activity [25], whereas the study SUGAR-DM-HF, which investigated the use of empagliflozin in patients with T2DM and HFrEF, showed a 28% reduction in NT-proBNP levels [19]. In addition, our study showed a significant decrease in symptomatic burden in patients receiving SGLT2i compared with patients not receiving SGLT2i, as measured by NYHA functional classification thus showing a strong concordance with observed echocardiographic and biochemical improvements.
A subanalysis of changes in parameters of interest from baseline to follow-up in HFrEF patients receiving an SGLT2 inhibitor showed that there were no significant differences in these parameters with respect to sex and DM status, whereas patients with nonischemic idiopathic cardiomyopathy appeared to have a greater numerical improvement in all parameters compared with patients with ischemic cardiomyopathy but without reaching statistical significance. Interestingly, a subanalysis of the study PARADIGM-HF, which focused on HFrEF etiology, showed a similar benefit of sacubitril/valsartan in both etiologic groups, although the risk reduction of adverse events was numerically greater in patients with nonischemic idiopathic cardiomyopathy than in patients with ischemic cardiomyopathy [26]. A benefit signal along the same lines was observed in the subanalysis of the DAPA-HF trial, which showed that dapagliflozin had a similar effect on cardiovascular death regardless of the type of heart failure, although the risk reduction was numerically greater in patients with nonischemic idiopathic cardiomyopathy than in patients with ischemic cardiomyopathy [27]. These findings are complementary to our observations noted in our HFrEF population treated with SGLT2i.
The demonstrated results of positive LV remodeling as measured by echocardiographic parameters, NT-proBNP levels, and improvement in functional status can be explained to some extent by complementary and synergic mechanisms of combined neurohumoral therapy with SGLT2i and ARNI with the rest of foundational therapies in HFrEF. It is an accepted concept that initiation and up-dosing of all four foundational therapies in HFrEF significantly reduce mortality and morbidity through additive effects [28]. Solomon et al performed a subanalysis of DAPA-HF patients taking ARNI with dapagliflozin and showed similar efficacy and safety regardless of using sacubitril/valsartan. They emphasized a possible complementary and additive effect of concomitant use of ARNI and SGLT2i use to reduce morbidity and mortality in patients with HFrEF [29]. A meta-analysis of 6 randomized controlled trials on the reduction of CV-mortality, all-cause mortality, and hospitalization for heart failure with SGLT2is in combination with ARNI compared with ARNI-monotherapy showed a similar effect on the primary endpoint, whereas the combination of SGLT2i and ARNI resulted in a better cardiovascular protective effect [30].
Several limitations should be noted in this study. First, this was a single-center study that involved the patient population at our institution. Although this may be a limitation, it is important to emphasize that all echocardiographic measurements were performed by the same echocardiographic specialist who was blinded to treatment assignment, thus reducing interobserver variability. Second, only a limited number of patients were included in each group, limiting the possibility that some of the results would have reached statistical significance if a larger number of patients had been included. Third, differences were found between the two groups in the presence of T2DM at baseline, but subanalysis for this characteristic did not show an interaction of diabetes mellitus with reported measures of interest, and diabetes mellitus was also not found as a significant independent predictor of the change in these outcomes as per linear regression analysis.
In conclusion, the addition of an SGLT2 inhibitor to sacubitril/valsartan and other maximally tolerated baseline therapies in HFrEF resulted in greater improvement of LV systolic function compared with a treatment regimen without SGLT2i at a 3-month follow-up. Outpatients with HFrEF who received SGLT2i showed significantly greater improvement in myocardial work index and global work efficiency at follow-up along with improvements in GLS, 3D LVEF, and NYHA status and achieved greater reductions in circulating NT-proBNP levels
Data Availability
Data sets generated and/or analyzed during the current study are available on reasonable request from the corresponding author.
Abbreviations
- ARNI:
-
Angiotensin receptor-neprilysin inhibitors
- BB:
-
Beta blocker
- CABG:
-
Coronary artery bypass grafting
- CRT:
-
Cardiac resynchronization therapy
- eGFR:
-
Estimated glomerular filtration rate
- GWI:
-
Global work index
- GCW:
-
Global constructive work
- GWW:
-
Global wasted work
- GWE:
-
Global work efficiency
- ICD:
-
Implantable cardioverter device
- LVEDV:
-
Left ventricular end-diastolic volume
- LV GLS:
-
Left ventricular global longitudinal strain
- LVESV:
-
Left ventricular end-systolic volume
- LVEF:
-
Left ventricular ejection fraction
- MRA:
-
Mineralocorticoid receptor antagonist
- NYHA:
-
New York Heart Assocciation
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- PCI:
-
Percutaneous coronary intervention
- TAPSE:
-
Tricuspid annular plane systolic excursion
References
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24. https://doi.org/10.1056/NEJMoa2022190.
Salah HM, Verma S, Santos-Gallego CG, Bhatt AS, Vaduganathan M, Khan MS, et al. Sodium-glucose cotransporter 2 inhibitors and cardiac remodeling. J Cardiovasc Transl Res. 2022;15(5):944–56. https://doi.org/10.1007/s12265-022-10220-5.
Xie Y, Wei Y, Li D, Pu J, Ding H, Zhang X. Mechanisms of SGLT2 inhibitors in heart failure and their clinical value. J Cardiovasc Pharmacol. 2023;81(1):4–14. https://doi.org/10.1097/fjc.0000000000001380.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Zhang N, Wang Y, Tse G, Korantzopoulos P, Letsas KP, Zhang Q, et al. Effect of sodium-glucose cotransporter-2 inhibitors on cardiac remodelling: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;28(17):1961–73. https://doi.org/10.1093/eurjpc/zwab173.
Lund LH, Pitt B, Metra M. Left ventricular ejection fraction as the primary heart failure phenotyping parameter. Eur J Heart Fail. 2022;24(7):1158. https://doi.org/10.1002/ejhf.2576.
Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26(2):185–91. https://doi.org/10.1016/j.echo.2012.10.008.
Lakatos BK, Ruppert M, Tokodi M, Olah A, Braun S, Karime C, et al. Myocardial work index: a marker of left ventricular contractility in pressure- or volume overload-induced heart failure. ESC Heart Fail. 2021;8(3):2220–31. https://doi.org/10.1002/ehf2.13314.
Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: a non-invasive index of myocardial work. Eur Heart J. 2012;33(6):724–33. https://doi.org/10.1093/eurheartj/ehs016.
Hubert A, Le Rolle V, Leclercq C, Galli E, Samset E, Casset C, et al. Estimation of myocardial work from pressure-strain loops analysis: an experimental evaluation. Eur Heart J Cardiovasc Imaging. 2018;19(12):1372–9. https://doi.org/10.1093/ehjci/jey024.
Abawi D, Rinaldi T, Faragli A, Pieske B, Morris DA, Kelle S, et al. The non-invasive assessment of myocardial work by pressure-strain analysis: clinical applications. Heart Fail Rev. 2022;27(4):1261–79. https://doi.org/10.1007/s10741-021-10119-4.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. https://doi.org/10.1093/eurheartj/ehw128.
Lan NSR, Fegan PG, Yeap BB, Dwivedi G. The effects of sodium-glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions. ESC Heart Fail. 2019;6(5):927–35. https://doi.org/10.1002/ehf2.12505.
Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc. 2020;9(9):e015716. https://doi.org/10.1161/JAHA.119.015716.
Bouali Y, Donal E, Gallard A, Laurin C, Hubert A, Bidaut A, et al. Prognostic usefulness of myocardial work in patients with heart failure and reduced ejection fraction treated by sacubitril/valsartan. Am J Cardiol. 2020;125(12):1856–62. https://doi.org/10.1016/j.amjcard.2020.03.031.
Valentim Gonçalves A, Galrinho A, Pereira-da-Silva T, Branco L, Rio P, Timóteo AT, et al. Myocardial work improvement after sacubitril–valsartan therapy: a new echocardiographic parameter for a new treatment. J Cardiovasc Med. 2020;21(3):223–30. https://doi.org/10.2459/jcm.0000000000000932.
Wang CL, Chan YH, Wu VC, Lee HF, Hsiao FC, Chu PH. Incremental prognostic value of global myocardial work over ejection fraction and global longitudinal strain in patients with heart failure and reduced ejection fraction. Eur Heart J Cardiovasc Imaging. 2021;22(3):348–56. https://doi.org/10.1093/ehjci/jeaa162.
Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143(6):516–25. https://doi.org/10.1161/CIRCULATIONAHA.120.052186.
Gamaza-Chulian S, Diaz-Retamino E, Gonzalez-Teston F, Gaitero JC, Castillo MJ, Alfaro R, et al. Effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors on left ventricular remodelling and longitudinal strain: a prospective observational study. BMC Cardiovasc Disord. 2021;21(1):456. https://doi.org/10.1186/s12872-021-02250-9.
Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, Videbaek L, et al. Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: a substudy of the empire hf randomized clinical trial. JAMA Cardiol. 2021;6(7):836–40. https://doi.org/10.1001/jamacardio.2020.6827.
Medvedofsky D, Maffessanti F, Weinert L, Tehrani DM, Narang A, Addetia K, et al. 2D and 3D echocardiography-derived indices of left ventricular function and shape: relationship with mortality. JACC Cardiovasc Imaging. 2018;11(11):1569–79. https://doi.org/10.1016/j.jcmg.2017.08.023.
Benameur N, Arous Y, Ben Abdallah N, Kraiem T. Comparison between 3D echocardiography and cardiac magnetic resonance imaging (cmri) in the measurement of left ventricular volumes and ejection fraction. Curr Med Imaging Rev. 2019;15(7):654–60. https://doi.org/10.2174/1573405614666180815115756.
Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, et al. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68(22):2425–36. https://doi.org/10.1016/j.jacc.2016.09.931.
Jensen J, Omar M, Kistorp C, Poulsen MK, Tuxen C, Gustafsson I, et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: a double-blinded, randomized, and placebo-controlled trial. Am Heart J. 2020;228:47–56. https://doi.org/10.1016/j.ahj.2020.07.011.
Balmforth C, Simpson J, Shen L, Jhund PS, Lefkowitz M, Rizkala AR, et al. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM-HF. JACC Heart Fail. 2019;7(6):457–65. https://doi.org/10.1016/j.jchf.2019.02.015.
Butt JH, Nicolau JC, Verma S, Docherty KF, Petrie MC, Inzucchi SE, et al. Efficacy and safety of dapagliflozin according to aetiology in heart failure with reduced ejection fraction: insights from the DAPA-HF trial. Eur J Heart Fail. 2021;23(4):601–13. https://doi.org/10.1002/ejhf.2124.
Borovac JA. Early in-hospital initiation and optimization of comprehensive disease-modifying pharmacotherapy in patients with heart failure with reduced ejection fraction: a time for the paradigm shift. Expert Rev Cardiovasc Ther. 2022;20(2):91–4. https://doi.org/10.1080/14779072.2022.2039626.
Solomon SD, Jhund PS, Claggett BL, Dewan P, Kober L, Kosiborod MN, et al. Effect of dapagliflozin in patients With HFrEF treated with sacubitril/valsartan: the DAPA-HF Trial. JACC Heart Fail. 2020;8(10):811–8. https://doi.org/10.1016/j.jchf.2020.04.008.
Yan Y, Liu B, Du J, Wang J, Jing X, Liu Y, et al. SGLT2i versus ARNI in heart failure with reduced ejection fraction: a systematic review and meta-analysis. ESC Heart Fail. 2021;8(3):2210–9. https://doi.org/10.1002/ehf2.13313.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ivona Mustapic and Darija Bakovic. The first draft of the manuscript was written by Ivona Mustapic, and all authors commented on previous versions of the manuscript. All authors read and approved the final version of the current manuscript.
Conceptualization: Ivona Mustapic; methodology: Ivona Mustapic and Darija Bakovic; formal analysis and investigation: Ivona Mustapic, Darija Bakovic, Zora Susilovic Grabovac, and Josip Andelo Borovac; data curation: Josip Andelo Borovac; project administration: Ivona Mustapic; supervision and validation: Zora Susilovic Grabovac; writing—original draft preparation: Ivona Mustapic; visualization: Ivona Mustapic; writing—review and editing: Ivona Mustapic, Josip Andelo Borovac, and Darija Bakovic; resources: Ivona Mustapic and Darija Bakovic
Corresponding author
Ethics declarations
Ethical Approval
The study was conducted from March 2021 to April 2022 at the Clinic for Heart and Vascular Diseases, University Hospital of Split, Croatia. All procedures performed in studies with human participants were in accordance with the principles of the 2013 Declaration of Helsinki and were approved by the Ethics Committee of the University Hospital of Split filed under the number 2181-147/01/06/M.S.-20-02.
Consent to Participate
Each potential participant was informed about the design and objectives of the study, and all included patients read and signed the informed consent form for participation.
Consent for Publication
Patients signed an informed consent regarding the publication of their data.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Marat Fudim oversaw the review of this article
Ivona Mustapic and Darija Bakovic contributed equally to this work.
Key Message: Patients receiving SGLT2i in addition to fully optimized HFrEF therapy consisting of ARNi, BB, and MRA improved LV systolic function more than non-recipients.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mustapic, I., Bakovic, D., Susilovic-Grabovac, Z. et al. Left Ventricular Systolic Function After 3 Months of SGLT2 Inhibitor Therapy in Heart Failure Patients with Reduced Ejection Fraction. J. of Cardiovasc. Trans. Res. 16, 987–998 (2023). https://doi.org/10.1007/s12265-023-10389-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10389-3