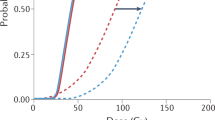
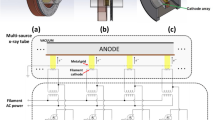
Abstract
FLASH radiotherapy (FLASH-RT) has great potential to improve patient outcomes. It delivers radiation doses at an ultra-high dose rate (UHDR: ≥ 40 Gy/s) in a single instant or a few pulses. Much higher irradiation doses can be administered to tumors with FLASH-RT than with conventional dose rate (0.01−0.40 Gy/s) radiotherapy. UHDR irradiation can suppress toxicity in normal tissues while sustaining antitumor efficiency, which is referred to as the FLASH effect. However, the mechanisms underlying the effects of the FLASH remain unclear. To clarify these mechanisms, the development of simulation models that can contribute to treatment planning for FLASH-RT is still underway. Previous studies indicated that transient oxygen depletion or augmented reactions between secondary reactive species produced by irradiation may be involved in this process. To discuss the possible mechanisms of the FLASH effect and its clinical potential, we summarized the physicochemical, chemical, and biological perspectives as well as the development of simulation modeling for FLASH-RT.

Similar content being viewed by others
Abbreviations
- BED:
-
Biologically effective dose
- CONV:
-
Conventional
- DSB:
-
Double-strand break
- GSH:
-
Glutathione
- IRT:
-
Independent reaction time
- LET:
-
Linear energy transfer
- LPO:
-
Lipid peroxidation
- LQ:
-
Linear-quadratic
- MD:
-
Molecular dynamics
- OER:
-
Oxygen enhancement ratio
- ROS:
-
Reactive oxygen species
- SBS:
-
Step-by-step
- SSB:
-
Single-strand break
- TOD:
-
Transient oxygen depletion
- UHDR:
-
Ultra-high dose rate
References
Kirby-Smith JS, Dolphin GW. Chromosome breakage at high radiation dose-rates. Nature. 1958;182:270–1.
Dewey DL, Boag JW. Modification of the oxygen effect when bacteria are given large pulses of radiation. Nature. 1959;183:1450–1.
Berry RJ, Hall EJ, Forster DW, Storr TH, Goodman MJ. Survival of mammalian cells exposed to X rays at ultra-high dose-rates. Br J Radiol. 1969;42:102–7.
Wilson JD, Hammond EM, Higgins GS, Petersson K. Ultra-high dose rate (FLASH) radiotherapy: silver bullet or fool’s gold? Front Oncol. 2020;9:1563.
Wilson P, Jones B, Yokoi T, Hill M, Vojnovic B. Revisiting the ultra-high dose rate effect: Implications for charged particle radiotherapy using protons and light ions. Br J Radiol. 2012;85:e933–9.
Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6:245ra93.
Vozenin MC, Fornel PD, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The advantage of flash radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res. 2019;25:35–42.
Levy K, Natarajan S, Wang J, Chow S, Eggold JT, Loo PE, et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci Rep. 2020;10:21600.
Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci USA. 2019;166:10943–51.
Montay-Gruel P, Acharya MM, Jorge PC, Petit B, Petridis IG, Fuchs P, et al. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin Cancer Res. 2021;27:775–84.
Friedl AA, Prise KM, Butterworth KT, Montay-Gruel P, Favaudon V. Radiobiology of the FLASH effect. Med Phys. 2022;49:1993–2013.
Favaudon V, Labarbe R, Limori CL. Model studies of the role of oxygen in the FLASH effect. Med Phys. 2022;49:2068–81.
Lin B, Huang D, Gao F, Yang Y, Wu D, Zhang Y, et al. Mechanisms of FLASH effect. Front Oncol. 2022;12: 995612.
Bogaerts E, Macaeva E, Isebaert S, Haustermans K. Molecular mechanisms behind the ultra-high dose rate “FLASH” effect. Int J Mol Sci. 2022;12: 995612.
Kacem H, Psoroulas S, Boivin G, Folkerts M, Grilj V, Lomax T, et al. Comparing radiolytic production of H2O2 and development of Zebrafish embryos after ultra high dose rate exposure with electron and transmission proton beams. Radiother Oncol. 2022;175:197–202.
Zlobinskaya O, Siebenwirth C, Greubel C, Hable V, Hertenberger R, Humble N, et al. The effects of ultra-high dose rate proton irradiation on growth delay in the treatment of human tumor xenografts in nude mice. Radiat Res. 2014;181:177–83.
Kim MM, Verginadis II, Goia D, Haertter A, Shoniyozov K, Zou W, et al. Comparison of FLASH proton entrance and the spread-out Bragg peak dose regions in the sparing of mouse intestinal crypts and in a pancreatic tumor model. Cancers. 2021;13:4244.
Rama N, Saha T, Shukla S, Goda C, Milewski D, Mascia AE, et al. Improved tumor control through T-cell infiltration modulated by ultra-high dose rate proton FLASH using a clinical pencil beam scanning proton system. Int J Radiat Oncol Biol Phys. 2019;105:S164–5.
Shukla S, Saha T, Rama N, Acharya A, Le T, Bian F, et al. Ultra-high dose-rate proton FLASH improves tumor control. Radiother Oncol. 2023;186: 109741.
Tinganelli W, Weber U, Purspitasari A, Simoniello P, Abdollahi A, Oppermann J, et al. FLASH with carbon ions: Tumor control, normal tissue sparing, and distal metastasis in a mouse osteosarcoma model. Radiother Oncol. 2022;175:185–90.
Vozenin M-C, Hendry JH, Limoli CL. Biological benefits of ultra-high dose rate FLASH radiotherapy: sleeping beauty awoken. Clin Oncol. 2019;31:407–15.
Limoli CL, Vozenin M-C. Reinventing radiobiology in the light of FLASH radiotherapy. Annu Rev Cancer Biol. 2023;7:1–23.
Bourhis J, Montay-Gruel P, Jorge PG, Bailat C, Petit B, Ollivier J, et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother Oncol. 2019;139:11–7.
Mascia AE, Daugherty EC, Zhang Y, Lee E, Xiao Z, Sertorio M, et al. Proton FLASH radiotherapy for the treatment of symptomatic bone metastases: The FAST-01 nonrandomized trial. JAMA Oncol. 2023;9:62–9.
Gaide O, Herrera F, Sozzi WJ, Jorge PG, Kinj R, Bailat C, et al. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother Oncol. 2022;174:87–91.
Bley CR, Wolf F, Jorge PG, Grilj V, Petridis I, Petit B, et al. Dose and volume limiting late toxicity of FLASH radiotherapy in cats with squamous cell carcinoma of the nasal planum and in minipigs. Clin Cancer Res. 2022;28:3814–23.
Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (·OH/·O-) in Aqueous Solution. J Phys Chem Ref Data. 1988;17:513–886.
Spitz DR, Buettner GR, Petronek MS, St-Aubin JJ, Flynn RT, Waldron TJ, et al. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol. 2019;139:23–7.
Adrian G, Konradsson E, Lempart M, Bäck S, Petersson K. The FLAH effect depends on oxygen concentration. Br J Radiol. 2020;93:2019072.
Zackrisson BU, Nystrom UH, Ostbergh P. Biological response in vitro to pulsed high dose rate electrons from a clinical accelerator. Acta Oncol. 1991;30:747–51.
Cygler J, Klassen NV, Ross CK, Bichay TJ, Raaphorst GP. The survival of aerobic and anoxic human glioma and melanoma cells after irradiation at ultrahigh and clinical dose rates. Radiat Res. 1994;140:79–84.
Adrian G, Ruan J-L, Paillas S, Cooper CR, Petersson K. In vitro assays for investigating the FLASH effect. Expert Rev Mol Med. 2022;24: e10.
Adrian G, Konradsson E, Beyer S, Wittrup A, Butterworth KT, McMahon SJ, et al. Cancer cells can exhibit a sparing FLASH effect at low doses under normoxic in vitro-conditions. Front Oncol. 2021;11: 686142.
Cao X, Zhang R, Esipova TV, Allu SR, Ashraf R, Rahman M, et al. Quantification of oxygen depletion during FLASH irradiation in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2021;111:240–8.
Jansen J, Knoll J, Beyreuther E, Pawelke J, Skuza R, Hanley R, et al. Does FLASH deplete oxygen? Experimental evaluation for photons, protons, and carbon ions. Med Phys. 2021;48:3982–90.
Jia M, Cao X, Pogue BW, Peng H. A mechanistic consideration of oxygen enhancement ratio, oxygen transport and their relevancies for normal tissue sparing under FLASH irradiation. Holistic Integrity Oncology. 2022;1:13.
Labarbe R, Hotoiu L, Barbier J, Favaudon V. A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother Oncol. 2020;153:303–10.
Michael BD, Davies S, Held KD. Ultrafast chemical repair of DNA single and double strand break precursors in irradiated V79 cells. Basic Life Sci. 1986;38:89–100.
Zakaria AM, Colangelo NW, Meesunguoen J, Azzam E, Plourde M-E, Jay-Gerin J-P. Ultra-high dose-rate, pulsed (FLASH) radiotherapy with carbon ions: Generation of early, transient, highly oxygenated conditions in the tumor environment. Radiat Res. 2020;194:578–93.
Weber UA, Scifoni E, Durante M. FLASH radiotherapy with carbon ion beams. Med Phys. 2022;49:1974–92.
Kreipl MS, Friedland W, Paretzke HG. Interaction of ion tracks in spatial and temporal proximity. Radiat Environ Biophys. 2009;48:349–59.
Ramos-Méndez J, Domínguez-Kondo N, Schuemann J, McNamara A, Moreno-Barbosa E, Faddegon B. LET-dependent intertrack yields in proton irradiation at ultra-high dose rates relevant for FLASH therapy. Radiat Res. 2020;194:351–62.
Alanazi A, Meesungnoen J, Jay-Gerin JP. A computer modeling study of water radiolysis at high dose rates. relevance to flash radiotherapy. Radiat Res. 2021;195:149–62.
Derksen L, Flatten V, Engenhart-Cabillic R, Zink K, Baumann K-S. A method to implement inter-track interactions in Monte Carlo simulations with TOPAS-nBio and their influence on simulated radical yields following water radiolysis. Phys Med Biol. 2023;68: 135017.
Baikalov A, Abolfath R, Schüler E, Mohan R, Wilkens JJ, Bartzsch S. Intertrack interaction at ultra-high dose rates and its role in the FLASH effect. Front Phys. 2023;11:1215422.
Caër SL. Water radiolysis: Influence of oxide surfaces on H2 production under ionizing radiation. Water (Basel). 2011;3:235–53.
Samuel AH, Magee JL. Theory of radiation chemistry. II. Track effects in radiolysis of water. J Chem Phys. 1953;21:1080–7.
Blain G, Vandenborre J, Villoing D, Fiegel V, Fois GR, Haddad F, et al. Proton irradiations at ultra-high dose Rate vs. Conventional Dose Rate: Strong Impact on Hydrogen Peroxide Yield. Radiat Res. 2022;198:318–24.
Kusumoto T, Kitamura H, Hojo S, Konishi T, Kodaira S. Significant changes in yields of 7-hydroxy-coumarin-3-carboxylic acid produced under FLASH radiotherapy conditions. RSC Adv. 2020;10:38709–14.
Kusumoto T, Inaniwa T, Mizushima K, Sato S, Hojo S, Kitamura H, et al. Radiation chemical yields of 7-hydroxy-coumarin-3-carboxylic acid for proton- and carbon-ion beams at ultra-high dose rates: Potential roles in FLASH effects. Radiat Res. 2022;198:255–62.
Fouillade C, Curras-Alonso S, Giuranno L, Quelennec E, Heinrich S, Bonnet-Boissinot S, et al. FLASH irradiation spares lung progenitor cells and limits the incidence of radio-induced senescence. Clin Cancer Res. 2020;26:1497–506.
Fradet-Turcotte A, Canny MD, Escribano-Díaz C, Orthwein A, Leung CCY, Huang H, et al. 53BP1 is a reader of the DNA-damage- induced H2A Lys 15 ubiquitin mark. Nature. 2013;499:50–4.
Buonanno M, Grilj V, Brenner DJ. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother Oncol. 2019;139:51–5.
Guo Z, Buonanno M, Harken A, Zhou G, Hei TK. Mitochondrial damage response and fate of normal cells exposed to FLASH irradiation with protons. Radiat Res. 2022;197:569–82.
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91.
Lennicke C, Rahn J, Lichtenfels R, Wessjohann LA, Seliger B. Hydrogen peroxide - Production, fate and role in redox signaling of tumor cells. Cell Commun Signal. 2015;13:39.
Perillo B, Donato MD, Pezone A, Zazzo ED, Giovannelli P, Galasso G, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192–203.
Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer Nat Res. 2022;22:280–97.
Salnikow K. Role of iron in cancer. Semin Cancer Biol. 2021;76:189–94.
Ohsawa D, Hiroyama Y, Kobayashi A, Kusumoto T, Kitamura H, Hojo S, et al. DNA strand break induction of aqueous plasmid DNA exposed to 30 MeV protons at ultra-high dose rate. Radiat Res. 2022;63:255–60.
Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: From mechanistic understanding to cancer treatment. DNA Repair. 2007;6:923–35.
Harper JV, Anderson JA, O’Neill P. Radiation induced DNA DSBs: Contribution from stalled replication forks? DNA Repair. 2010;9:907–13.
Thompson LH. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: The molecular choreography. Mutat Res. 2012;751:158–246.
Tashiro M, Yoshida Y, Oike T, Nakao M, Yusa K, Hirota Y, et al. First human cell experiments with FLASH carbon ions. Anticancer Res. 2022;42:2469–77.
Khan S, Bassenne M, Wang J, Manjappa R, Melemenidis S, Breitkreutz DY, et al. Multicellular spheroids as in vitro models of oxygen depletion during FLASH irradiation. Int J Radiat Oncol Biol Phys. 2021;110:833–44.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85.
Vilaplana-Lopera N, Abu-Halawa A, Walker E, Kim J, Moon EJ. Ferroptosis, a key to unravel the enigma of the FLASH effect? Br J Radiol. 2022;95:2022085.
Froidevaux P, Grilj V, Bailat C, Geyer WR, Bochud F, Vozenin MC. FLASH irradiation does not induce lipid peroxidation in lipids micelles and liposomes. Radiat Phys Chem. 2023;205: 110733.
Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–62.
Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–85.
Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS, et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem Biol. 2020;15:469–84.
Boscolo D, Schfoni E, Durante M, Krämer M, Fuss MC. May oxygen depletion explain the FLASH effect? A chemical track structure analysis. Radiother Oncol. 2021;162:68–75.
Lai Y, Jia X, Chi Y. Modeling the effect of oxygen on the chemical on the chemical stage of water radiolysis using GPU-based microscopic Monte Carlo simulations, with an application in FLASH radiotherapy. Phys Med Biol. 2021;66: 025004.
Zhu H, Li J, Deng X, Qiu R, Wu Z, Zhang H. Modeling of cellular response after FLASH irradiation: A quantitative analysis based on the radiolytic oxygen depletion hypothesis. Phys Med Biol. 2021;66: 185009.
Hu A, Qiu R, Wu Z, Zhang H, Li J. CPU-GPU coupling independent reaction times method in NASIC and application in water radiolysis by FLASH irradiation. Biomed Phys Eng Express. 2022;8: 025015.
Espinosa-Rodriguez A, Sanchez-Parcerisa D, Ibáñez P, Vera-Sánchez JA, Mazal A, Fraile LM, et al. Radical production with pulsed beams: understanding the transition to FLASH. Mol Sci. 2022;23:13484.
Abolfath R, Grosshans D, Mohan R. Oxygen depletion in FLASH ultra-high-dose-rate radiotherapy: A molecular dynamics simulation. Med Phys. 2020;47:6551–61.
Abolfath R, Baikalov A, Bartzsch S, Afshordi N, Mohan R. The effect of non-ionizing excitations on the diffusion of ion species and inter-track correlations in FLASH ultra-high dose rate radiotherapy. Phys Med Biol. 2022;67: 105005.
Pratx G, Kapp DS. A computational model of radiolytic oxygen depletion during FLASH irradiation and its effect on the oxygen enhancement ratio. Phys Med Biol. 2020;65: 109501.
Zhou S, Zheng D, Fan Q, Yan Y, Wang S, Lei Y, et al. Minimum dose rate estimation for pulsed FLASH radiotherapy: A dimensional analysis. Med Phys. 2020;47:3243–9.
Petersson K, Adrian G, Butterworth K, McMahon SJ. A quantitative analysis of the role of oxygen tension in FLASH radiation therapy. Int J Radiat Oncol Biol Phys. 2020;107:539–47.
Rothwell BC, Kirkby NF, Merchant MJ, Chadwick AL, Lowe M, Mackay RI, et al. Determining the parameter space for effective oxygen depletion for FLASH radiation therapy. Phys Med Biol. 2021;66: 055020.
Hu A, Qiu R, Wu Z, Li WB, Li J. A computational model for oxygen depletion hypothesis in FLASH effect. Radiat Res. 2022;2022:135–83.
Cui S, Pratx G. 3D computational model of oxygen depletion kinetics in brain vasculature during FLASH RT and its implications for in vivo oximetry experiments. Med Phys. 2021;49:3914–25.
Taylor E, Hill RP, Létourneau D. Modeling the impact of spatial oxygen heterogeneity on radiolytic oxygen depletion during FLASH radiotherapy. Phys Med Biol. 2022;67: 115017.
Liew H, Mein S, Tessonnier T, Abdollahi A, Debus J, Dokic I, et al. Do we preserve tumor control probability (TCP) in FLASH radiotherapy? A model-based analysis Mol Sci. 2023;24:5118.
Song H, Kim Y, Sung W. Modeling of the FLASH effect for ion beam radiation therapy. Phys Med. 2023;108: 102553.
Jin JY, Gu A, Wang W, Oleinick NL, Machtay M, Kong FM. Ultra-high dose rate effect on circulating immune cells: A potential mechanism for FLASH effect? Radiother Oncol. 2020;149:55–62.
Cucinotta FA, Smirnova OA. Effects of flash radiotherapy on blood lymphocytes in humans and small laboratory animals. Radiat Res. 2023;199:240–51.
Camazzola G, Boscolo D, Scifoni E, Dorn A, Durante M, Krämer M, et al. TRAX-CHEMxt: Towards the homogeneous chemical stage of radiation damage. Mol Sci. 2023;24:9398.
Yao N, Chen X, Fu ZH, Zhang Q. Applying classical, ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries. Chem Rev. 2022;122:10970–1021.
Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review1. Cancer Res. 1989;49:6449–65.
Filatenkov A, Baker J, Mueller AMS, Kenkel J, Ahn GO, Dutt S, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21:3727–39.
Acknowledgements
The authors thank Dr Hiroyuki Date (Hokkaido University) for helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Shiraishi, Y., Matsuya, Y. & Fukunaga, H. Possible mechanisms and simulation modeling of FLASH radiotherapy. Radiol Phys Technol 17, 11–23 (2024). https://doi.org/10.1007/s12194-023-00770-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-023-00770-x