Abstract
The COVID-19 pandemic has resulted in a large increase in the number of patients admitted to hospitals. Radiological technologists (RTs) are often required to perform portable chest X-ray radiography on these patients. Normally, when performing a portable X-ray, radiation protection equipment is critical as it reduces the scatter radiation dose to hospital workers. However, during the pandemic, the use of a lead shield caused a heavy weight burden on workers who were responsible for a large number of patients. This study aimed to investigate scatter radiation doses received at various distances, directions, and positions. Radiation measurements were performed using the PBU-60 whole body phantom to determine scatter radiation doses at 100–200 cm and eight different angles around the phantom. The tests were conducted with and without lead shielding. Additionally, the doses were compared using the paired t test (p < 0.005) to determine suitable positions for workers who did not wear lead protection that adhered to radiation safety requirements. Scatter radiation doses of all 40 tests showed a highest and lowest value of 1285.5 nGy at 100 cm in the anteroposterior (AP) semi upright position and 134.7 nGy at 200 cm in the prone position, respectively. Correlation analysis between the dosimeter measurement and calculated inverse square law showed good correlation, with an R2 value of 0.99. Without lead shielding, RTs must stay at a distance greater than 200 cm from patients for both vertical and horizontal beams to minimize scatter exposure. This would allow for an alternative way of performing portable chest radiography for COVID-19 patients without requiring lead shielding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In December 2019, the coronavirus disease 2019 (COVID-19) emerged in Wuhan, China, and spread rapidly through human-to-human transmission. COVID-19 has since become a globally recognized pandemic declared by the World Health Organization (WHO); the disease presents as a severe acute respiratory syndrome and causes death [1,2,3,4]. The WHO also declared COVID-19 to be a public health emergency of international concern requiring international cooperation and enforcement of a quarantine policy [1, 2]. Currently, safe and effective vaccines for controlling the COVID-19 pandemic have been approved and made available by the WHO, including those developed by Pfizer, Moderna, AstraZeneca, Johnson & Johnson, and Sinopharm, which have helped decrease the number of infected patients, severity of symptoms, and rate of mortality. Meanwhile, the virus continues to evolve through one or more mutations, which leads to the possibility of ineffectiveness of COVID-19 vaccines [5, 6]. Therefore, screening and diagnostic test protocols for COVID-19 are still required. The reverse transcription polymerase chain reaction (RT-PCR) test has been recommended by the Centers for Disease Control (CDC) and the WHO, and is considered as the gold standard method for COVID-19 screening [2, 3, 7]. However, RT-PCR has limitations of long testing times, need for specific medical staff, and high costs, making it difficult to support the millions at risk [2, 3]. Many studies have suggested the use of chest computed tomography (CT) due to its high sensitivity and specificity, especially in asymptomatic patients at an early stage. However, the challenges of using CT during a global pandemic has raised concerns in many lower- to middle-income countries due to its high cost, the requirement for specialist expertise, and scanner availability [2, 7]. Moreover, performing CT scans may raise radiation exposure concerns, and decontaminating the CT scanner/equipment following use by COVID-19 patients is time consuming [2, 8]. Thus, the widely available usage of chest X-rays in most healthcare institutes may be a cost-effective option for quick diagnosis [3, 7, 8]. For observing and isolating patients in infection-controlled areas, portable X-rays play an important role in both diagnosis and follow-up by avoiding unnecessary transportation of infected patients, thereby reducing the possibility of spreading infection [7, 9,10,11]. On the other hand, the advantage of portable X-rays comes at the cost of increased radiation exposure to hospital staff because radiation protective systems are not equivalent to those in radiology departments. Radiation received from portable chest X-rays is considered to be low dose, but without proper shielding, hospital workers should be concerned about accumulative doses, which could lead to severe effects over the long term [12,13,14]. According to the International Commission on Radiological Protection (ICRP), the effective dose limit for the whole body is 100 mSv over five consecutive years and 50 mSv in any 1 year for occupational workers [15]. Therefore, a radiological technologist (RT) and assistant who control portable X-ray machines must wear, or stay behind, high attenuation material shielding (i.e., lead, bismuth, or barium sulfate) temporarily during image acquisition to achieve a dosage that is as low as reasonably achievable (ALARA), thus minimizing radiation exposure [14, 16,17,18]. During COVID-19 epidemics, an RT entering infected zones is required to wear personal protective equipment (PPE) while a second worker waits outside the zone to remove cassettes without touching the wrapped outer layers, to minimize contamination [10]. The large number of portable chest X-rays taken during the COVID-19 epidemic has increased the workloads of technicians. In practice, a hospital worker may skip the use of lead shields to avoid their weight, and instead move further away from the X-ray source. Therefore, a worker’s received radiation from portable chest X-rays is an interesting issue that has been investigated by many researchers, including ourselves [19,20,21]. The aim of the present study was to quantify the scatter radiation exposure from portable chest X-ray examinations with and without protective shielding to compare the doses and to identify a suitable distance/angle between the worker and the radiation source, without shielding, to confirm worker safety while performing duties with COVID-19 patients.
2 Materials and methods
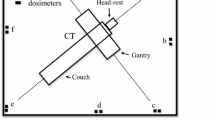
This study examined the exposure technique parameters (kVp, mAs, SID) used over a 3-month period for portable chest X-ray examinations (posteroanterioir (PA) upright, anteroposterior (AP) semi upright, AP supine, prone) performed by two portable X-ray units in Paolo Kaset Hospital, Thailand: Shenzhen Angell portable, China (S/N OW32101233L) and Hitachi Sirius portable, Japan (S/N SX1440331). Assessment of these techniques was performed using the most common exposure technique values used to estimate the appropriate parameters for performing this experiment. This study used the scatter radiation from a MUX-10 version 1.00 X-ray portable device, Shimadzu, Japan (S/N 3YCFC6B6C009) that was setup in an X-ray room at Rangsit University, Thailand for all experiments. The calibrated Geiger–Muller (GM) counter model 14C (S/N 339236) by secondary standard dosimetry laboratory (certificate no. SM1291/260821 on 24 August 2021) was used to measure the background radiation for about 20 min in the X-ray room. The X-ray tube was positioned facing the chest of the whole body phantom with a 180-cm distance between the X-ray tube and phantom. A whole body phantom PBU-60 (Kyoto Kagaku, Japan) with a height of 165 cm and weighing 50 kg made using urethane-based resin, including bone/air composition with photon attenuation equivalent to a human, represented the patient. The phantom was placed on a table at a height of 75 cm above the floor in all four routine chest X-ray positions: PA upright, AP semi upright, AP supine, and prone. For the AP semi upright and AP supine positions, the image acquisition of the phantom was setup as for a normal patient. For the PA upright position, a PVC plastic-sealed barrier comprising four large clear plastic drapes (90 × 180 cm) with a 0.3 mm thickness, supported with PVC pipes, was installed around the phantom and positioned near a portable X-ray machine outside it. The setup was based on the clinical contamination minimization procedures for suspected COVID-19 walk-in patients at Paolo Kaset Hospital, Thailand. The ventilator tube was attached to the phantom in the prone position setup to simulate the conditions of COVID-19 patients with acute respiratory distress syndrome. The measurements were made using the most common clinical technical parameters 90 kV and 5 mAs for voltage and current, respectively. Scatter radiation was detected with a solid-state radiation dosimeter model AGMS-D + (diagnostic range), Radcal Accu-gold plus, USA (S/N 48–0688), which provided a real-time display. The solid-state dosimeter was calibrated using a secondary standard dosimetry laboratory (certificate no. 0462062452 on 5 March 2020) before use in this experimental study. The dosimeter probe was installed at a fixed height of 120 cm to demonstrate the whole body effective dose corresponding to the height of the highest dose from the results reported by Antonio et al. [21]. Figure 1 shows that the first measurement was taken at a 0-degree angle (0° corresponds to the median sagittal line in the direction of the X-ray tube) and a 100-cm distance between the mid-sternum of the phantom and the detector. The detector was repositioned in increments of 25 cm up to 200 cm as shown in Fig. 2, which is the standard position of the RT operating the X-ray machine. The scatter dose measurements were repeated for different directions around the phantom to obtain the scatter dose distribution map over 360°: the detector was changed in 45° increments (Fig. 3). The basic interpolation Eq. 1 was used to deduce the value between two points in a set of data to provide the scatter radiation dose in 1° increments over 360°, where x and y are the desired values, x1 and y1 are the input and output from the lower value, and x2 and y2 are the input and output from the higher value, respectively:
Each clinical simulation was setup with and without lead providing 0.5 mm protection shielding (UniRay, India). Three duplicate measurements were made for each measurement position and recorded. The scatter radiation dose levels with and without the lead shielding at various distances were compared using Eq. 2, considering the percentage dose difference [22]. Moreover, all results at different chest X-ray positions, distances, and directions were analyzed using the independent Student’s paired t test. Statistical significance was defined by a p value < 0.05 (deemed to be significant at a 95% confidence interval) [23].
where DW and DWO are the scatter radiation doses measured using the nGy unit at any distance with and without lead shielding, respectively.
3 Results
Clinical exposure parameters of portable chest X-rays collected from 4,600 patients suspected of or diagnosed with COVID-19 were obtained from the hospital in four positions (Fig. 4): PA upright (n = 2622), AP semi upright (n = 1104), AP supine (n = 644), and prone (n = 230). A typical parameter was obtained at 90 kVp, 5 mAs, and a source-to-image distance (SID) equal to 180 cm with a field size of 14 × 17 inches for average-sized patients. The average indoor background radiation dose in the X-ray room was 0.07 μGy/h as shown on the GM counter. Table 1 and Fig. 5 report the scatter radiation dose measured at 100–200 cm when a 0.5-mm lead shielding was inserted and removed between the radiation source and the dosimeter. When shielding was present, the scatter radiation dose was only detected at a 100-cm distance for all measurements. At a greater distance, no dose was detectable due to the very low radiation penetrating the lead shielding. In cases without lead shielding, the maximum average dose values (1.29 µGy) for chest X-ray examinations were found at the nearest distance of 100 cm for the chest AP in the semi upright position. It was observed that radiation doses detected at 200 cm for the chest prone position was the lowest, corresponding to 10.48% of the maximum dose. Dose variation depends on the distance from the X-ray machine, with the observed doses decreasing as experimental distances increase. A comparison of the doses with and without lead shielding showed a statistically significant difference at all distances for the four positions.
Scatter radiation doses per examination without 0.5-mm lead shielding at 100–200 cm from the mid-sternum of the PBU-60 phantom at eight different angles are shown in the dose color map for AP semi upright, PA upright, AP supine, and prone chest X-ray positions in Fig. 6a, b, c, and d, respectively. Considering the scattered radiation dose rate readings around the phantom, the maximum dose was achieved at 90° then decreased slightly at different angles and reached the minimum value at 270° for the AP semi upright and PA upright positions. For the AP supine and prone positions, the maximum dose was achieved at the 45˚ and 135° positions, respectively, and were approximately the same and reached minimum values at 180°.
Furthermore, theoretical inverse square law values were calculated to indicate the accuracy and precision of the measurements for all positions. Comparisons of scatter radiation doses between the dosimeter-measured and theoretical values indicated good correlation with R2, approximately equal to 0.99, as shown in Fig. 7.
4 Discussion and conclusion
All medical institutions in Thailand have had a large number of COVID-19 patients, and RTs are responsible for performing chest X-rays on those suspected and diagnosed with COVID-19. The use of a portable X-ray machine is a potential method of acquiring X-ray images. Portable chest X-ray radiography is commonly performed in the AP semi upright and AP supine positions under normal conditions. However, for COVID-19, uncommon positioning for portable chest radiography, including PA upright and prone, were increasingly observed. Previous studies have investigated the scattered radiation dose from portable chest X-rays only in the AP semi upright and AP spine positions [20, 21, 24]. Therefore, information on the scattered dose from portable chest X-rays performed in the PA upright and prone positions is limited. In addition, during the COVID-19 pandemic, the number of patients who underwent portable chest X-rays was greatly increased (by approximately 10 times); in normal situations, approximately 140 cases/month with lead shielding have been observed whereas the COVID-19 pandemic resulted in approximately 1500 cases/month without lead shielding, which may have exceeded occupational dose limits. We, therefore, decided to investigate scatter radiation doses in all four positions for portable chest X-rays to determine the efficiency of radiation protection for RTs who remained in the room when lead shielding was unavailable or to reduce the weight burden, time required, and the number of equipment disinfection procedures. In this study, the background radiation dose, which was considered negligible due to the lowest dose rate seen in the experiment, was approximately 27 times higher than the background dose. The observed scatter radiation dose with a 0.5-mm lead shielding thickness could be detected only at 100 cm due to a single exposure in the AP semi upright, PA upright, AP supine, and prone positions; when the distance was increased, the scatter radiation could not be observed due to the values being below a minimal limitation range of the dosimeter (less than 20 nGy/s) [20]. Therefore, when the RT used a lead shield and stayed at a distance greater than 100 cm during the acquisition of portable chest X-rays, the radiation exposure was considered to be extrinsic. In contrast, the average dose without lead protection at 100 cm was 11 times higher than with lead protection and was further minimized by greater distances corresponding to the inverse square law theory. As expected, the use of lead protection devices reduced the amount of scatter radiation. Therefore, without lead protection, the safety of RTs who use portable chest X-ray machines cannot be guaranteed. However, according to the clinical settings in this study modeled using PBU-60 whole body phantom measurements, hospital workers must stay at least 200 cm away from the radiation source for lower radiation potential and increased safety.
Scattered radiation exposure provided with a vertical beam (AP supine and prone positions) was generally lower than that with a horizontal beam (AP semi upright and PA upright), consistent with the study conducted by Duetting et al. [25]. The results were affected by the X-ray tube directionality as the vertical beam X-ray tube faced the floor while the horizontal beam faced the opposite wall [20]. According to Burrage et al., [26] the maximum scatter radiation yield at 1 m from the phantom is 0.05 µSv. This differs from the results of the present study, which recorded a maximum radiation of approximately 1.29 µSv. Slight differences with respect to previous studies are also attributable to the superior exposure techniques of this study (90 kVp, 5 mAs), which is different from the Burrage et al. study (60 kVp, 3.2 mAs). In terms of other differences, Burrage et al. used a newborn phantom while the present study used an adult whole body phantom that produced more patient scattering. Additionally, the increase in kVp may be due to a transition from the photoelectric effect to the Compton scattering interaction, which led to increased scatter radiation doses, consistent with the findings of Antonio et al. [21]. Renger et al. [24] reported that scatter radiation changed in direct proportion to voltages (high kV produces high scatter). For angular scatter radiation measurements, our study demonstrated a non-uniform distribution in eight dimensions with the maximum value of the horizontal beam set at 90°, which probably arose from the primary radiation source facing directly toward the 90° position and the opposite site at 270°, resulting in the detection of minimum scatter radiation. Thus, it is important to note that RTs should avoid standing at direct radiation intensity positions as also suggested in the Lee et al. study, which measured scatter radiation doses from C-arm fluoroscopy. In addition, inhomogeneous doses at different directions were probably caused by the irregular geometry of the phantom. A study by Trinh et al. [27] found that the maximum scatter dose occurred at 135° for horizontal beam measurements. Longo et al. [28] measured the scatter dose for vertical beams at 0°, 45°, 90°, 135°, 180°, 225°, 270°, and 315°, and the authors obtained the greatest value at 0° and lowest value at 180°. Our results agree with the previous study for the lowest value of the vertical beam. However, the maximum values for the vertical and horizontal beams in the present study were at 45° and 90°, respectively, which differs from the conclusions of Trinh et al. and Longo et al., both of whom reported differing results caused by other factors, including the use of a different phantom, the measurement setup, heel effect phenomena, angular evaluation, and machine geometry. Assuming 250 working days per year (5 days per week and 50 weeks per year) and 50 portable chest X-rays per day, standing at 100 cm and 90°, the maximum RT exposure dose when wearing lead protection would be 1.82 mSv/year and 16.07 mSv/year when not wearing lead protection. Both these values are below the annual occupational dose limit, which must not exceed 20 mSv/ year. However, when the distance was increased to 200 cm without lead protection, the annual radiation exposure decreases to 1.68 mSv/year, which is a significant drop, and is close to the dose seen when wearing lead protection.
Finally, this study has some limitations. All experiments were conducted using only one portable X-ray machine and one radiation dosimeter. Additionally, scatter radiation doses are affected by anatomy and body size. The present work used a standard size PBU-60 whole body phantom to represent the patient, but the resulting scatter dose may be underestimated for a larger patient [29]. Moreover, the farthest distance for measurements was only 200 cm, and this was still unable to reach a similar scatter dose under the lead shielding condition. Instead of measurements, we extrapolated the scatter dose for the condition without lead shielding via the inverse square law, which produced the closest value to lead shielding, and was found at distances of 330, 270, 285, and 305 cm for chest X-rays in the AP semi upright, PA upright, AP supine, and prone positions, respectively.
In summary, radiation protection is necessary for portable X-ray examinations to diminish the quantities of radiation exposure. Exposure levels when wearing lead protection were indicated only at a distance of 100 cm and the dose at increased distances was negligible, resulting in greater radiation protection to RTs performing examinations. Quantitative information on radiation exposure levels due to scatter radiation for RTs acquiring chest X-rays in the AP supine and prone positions (vertical beam) delivered a 30% lower scatter radiation dose than in the AP semi upright and PA upright positions (horizontal beam). Based on these results and the inverse square law theory, we suggest that hospital workers should stay more than 100 cm away from the exposure field or as far away as possible when using portable X-ray machines to minimize radiation doses. During the COVID-19 pandemic, RTs were not required to wear lead shielding if they stayed at distances of 200 cm or greater, which resulted in similar scatter radiation dose values compared to the use of lead shielding. More importantly, dosimetric outcome data also provide information on scatter radiation dose distributions in clinical settings, which suggests that workers should avoid being at primary radiation directions and should stay at the opposite site of the primary beam, which would result in the lowest dose of all directions around the patient. In addition, our results showed that the doses RTs received from portable chest X-rays did not reach occupational dose limits recommended by the ICRP, assuming 12,500 chest X-ray portable examinations per year. Finally, these results may be useful for multi-disciplinary staff who are involved in portable X-ray examinations.
References
Urmi Patel NR, Chaudhary B, Patel R, Devdhara E, Rathod M. A detailed essay on the pandemic COVID-19. Indian J Public Health Res Dev. 2020;11(10):68–75.
Fields BKK, Demirjian NL, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19) diagnostic technologies: a country-based retrospective analysis of screening and containment procedures during the first wave of the pandemic. Clin Imaging. 2020;67:219–25.
Misra S, Jeon S, Lee S, et al. Multi-channel transfer learning of chest X-ray images for screening of COVID-19. Electronics. 2020;9(9):1388.
Gondauri D, Mikautadze E, Batiashvili M. Research on COVID-19 virus spreading statistics based on the examples of the cases from different countries. Electron J Gen Med. 2020;17(4):em209.
Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non–COVID-19 mortality risk—seven integrated health care organizations, United States, December 14, 2020–July 31, 2021. Morb Mortal Wkly Rep. 2021;70(43):1520.
Shekhar R, Garg I, Pal S, et al. COVID-19 vaccine booster: to boost or not to boost. Infect Dis Rep. 2021;13(4):924–9.
Xin Li CLaDZ. COVID-MobileXpert: on-device COVID-19 screening using snapshots of chest X-Ray. p. 1063–7. arXiv: 2004.03042.
Chun-Fu Yeh H-TC, Andy Wei, Hsin-Ming Chen, Po-Chen Kuo, Keng-Chi Liu, Mong-Chi Ko, Ray-Jade Chen, Po-Chang Lee, Jen-Hsiang Chuang, Chi-Mai Chen, Yi-Chang Chen,, Wen-Jeng Lee, Ning Chien, Jo-Yu Chen, Yu-Sen Huang, Yu-Chien Chang, Yu-Cheng Huang, Nai-Kuan Chou, Kuan-Hua Chao, Yi-Chin Tu, Yeun-Chung Chang, and Tyng-Luh Liu. A Cascaded Learning Strategy for Robust COVID-19 Pneumonia Chest X-Ray Screening. Electrical Eng Syst Sci. 2020;2:1–14. arXiv: 2004.12786.
Ciotti M, Ciccozzi M, Terrinoni A, et al. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57(6):365–88.
Mohakud S, Ranjan A, Naik S, et al. COVID-19 preparedness for portable x-rays in an Indian hospital—safety of the radiographers, the frontline warriors. Radiography (Lond). 2020;26(3):270–1.
Jacobi A, Chung M, Bernheim A, et al. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging. 2020;64:35–42.
Tochaikul G, Danthanavat N, Pilapong C, et al. Effect of low dose radiation from general X-ray to T-cell lymphocyte expression using an in vitro method. Radiat Effects Defects Solids. 2022:1–9.
Tochaikul G, Phattanasub A, Khemkham P, et al. Radioactive waste treatment technology: a review. Kerntechnik. 2022;87(2):208–25.
Nelson EM, Monazzam SM, Kim KD, et al. Intraoperative fluoroscopy, portable X-ray, and CT: patient and operating room personnel radiation exposure in spinal surgery. Spine J. 2014;14(12):2985–91.
Protection R. ICRP publication 103. Ann ICRP. 2007;37(2.4):2.
Tochaikul G, Moonkum N, Sriwongta S, Neamchumnan M, Thawornnittayakul A, Danthanavat N. Determination of appropriate proportional in-house flexible radiation shielding material using bismuth powder and natural-silicon rubber compounds. JCurr Sci Technol. 2021;11(2):277–86.
Danthanavat N, Mongkolsuk M, Tochaikul G, et al. Study of epoxy shielding material with barium sulphate for development of radiation protection materials in low-dose diagnostic X-ray. Radiat Eff Defects Solids. 2021;176(9–10):887–95.
Moonkum N, Pilapong C, Daowtak K, et al. Evaluation of silicone rubber shielding material composites enriched with BaSO4 and Bi2O3 particles for radiation shielding properties. Mater Res Innov. 2022. https://doi.org/10.1080/14328917.2022.2141953.
Chiang HW, Liu YL, Chen TR, et al. Scattered radiation doses absorbed by technicians at different distances from X-ray exposure: experiments on prosthesis. Biomed Mater Eng. 2015;26(Suppl 1):S1641–50.
Brady Z, Scoullar H, Grinsted B, et al. Technique, radiation safety and image quality for chest X-ray imaging through glass and in mobile settings during the COVID-19 pandemic. Phys Eng Sci Med. 2020;43(3):765–79.
AntonioAbrantes CR. Patrick sousa and sonia rodrigues. Scatter radiation exposure during mobile X-ray examinations. HealthManagementorg. 2017;17(1):67–72.
Moonkum N, Turathong S, Pinitpatcharalert A, et al. A phamtom study: In vivo rectal dosimetry of high dose rate brachytherapy in cervical cancer. Appl Radiat Isotopes. 2022;192:110604.
Kim TK. T test as a parametric statistic. Korean J Anesthesiol. 2015;68(6):540–6.
Renger B, Brieskorn C, Toth V, et al. Evaluation of dose reduction potentials of a novel scatter correction software for bedside chest X-ray imaging. Radiat Prot Dosimetry. 2016;169(1–4):60–7.
Duetting T, Foerste B, Knoch T, et al. Radiation exposure during chest X-ray examinations in a premature intensive care unit: phantom studies. Pediatr Radiol. 1999;29(3):158–62.
Burrage JW, Rampant PL, Beeson BP. Scatter and transmission doses from several pediatric X-ray examinations in a nursery. Pediatr Radiol. 2003;33(10):704–8.
Trinh AM, Schoenfeld AH, Levin TL. Scatter radiation from chest radiographs: is there a risk to infants in a typical NICU? Pediatr Radiol. 2010;40(5):704–7.
Longo M, Genovese E, Donatiello S, et al. Quantification of scatter radiation from radiographic procedures in a neonatal intensive care unit. Pediatr Radiol. 2018;48(5):715–21.
Lee K, Lee KM, Park MS, et al. Measurements of surgeons’ exposure to ionizing radiation dose during intraoperative use of C-arm fluoroscopy. Spine. 2012;37(14):1240–4.
Acknowledgements
The authors would like to acknowledge the Faculty of Radiological Technology, Rangsit University for the use of the lab institute and facilities for this study.
Funding
This project was financially supported by scholarships from the Research Institute of Rangsit University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Moonkum, N., Jitchom, S., Sukaram, S. et al. Determination of scattered radiation dose for radiological staff during portable chest examinations of COVID-19 patients. Radiol Phys Technol 16, 85–93 (2023). https://doi.org/10.1007/s12194-023-00698-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-023-00698-2