Abstract
Purpose
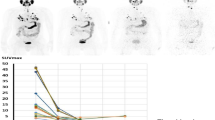
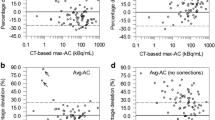
The objective of this study is to evaluate the lesion absorbed dose (AD), biological effective dose (BED), and equivalent uniform dose (EUD) to clinical–response relationship in lesional dosimetry for 131I therapy.
Methods
Nineteen lesions in four patients with metastatic differentiated thyroid cancer (DTC) were evaluated. The patients underwent PET/CT imaging at 2 h, 24 h, 48 h, 72 h, and 96 h post administration of ~ 33–65 MBq (0.89–1.76 mCi) of 124I before undergoing 131I therapy. The 124I PET/CT images were used to perform dosimetry calculations for 131I therapy. Lesion dose–rate values were calculated using the time–activity data and integrated over the measured time points to obtain AD and BED. The Geant4 toolkit was used to run Monte Carlo on spheres the same size as the lesions to estimate EUD. The lesion AD, BED, and EUD values were correlated with response data (i.e. change in lesion size pre- and post-therapy): complete response (CR, i.e. disappearance of the lesion), partial response (PR, i.e. any decrease in lesion length), stable disease (SD, i.e., no change in length), and progressive disease (PD, i.e., any increase in length).
Results
The lesion responses were CR and PR (58%, 11/19 lesions), SD (21%, 4/19), and PD (21%, 4/19). For CR and PR lesions, the ADs, BEDs and EUDs were > 75 Gy for 82% (9/11) and < 75 Gy for 18% (2/11). The ADs and BEDs were < 75 Gy for SD and PD lesions.
Conclusion
By performing retrospective dosimetry calculations for 131I therapy based on 124I PET/CT imaging, we evaluated the correlation of three dosimetric quantities to lesional response. When lesion AD, BED, and EUD values were > 75 Gy, 47% (9/19) of the lesions had a CR or PR. The AD, BED, and EUD values for SD and PD lesions were < 75 Gy. The data presented herein suggest that the greater the lesion AD, BED, and/or EUD, the higher the probability of a therapeutic response to 131I therapy.





Similar content being viewed by others
Availability of Data and/or Code Availability
Data and code data are available upon request from the authors.
References
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
Van Nostrand D, Atkins F, Yeganeh F, Acio E, Bursaw R, Wartofsky L. Dosimetrically determined doses of radioiodine for the treatment of metastatic thyroid carcinoma. Thyroid. 2002;12(2):121–34.
Atkins F, Van Nostrand D, Wartofsky L. Dosimetrically determined prescribed activity of I-131 for the treatment of metastatic differentiated thyroid cancer. In: Wartofsky L, Van Nostrand D, editors. Thyroid cancer: a comprehensive guide to clinical management. Berlin: Springer; 2016. p. 635–50.
Benua RS, Cicale NR, Sonenberg M, Rawson R. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am J Roentgenol Radium Therapy Nucl Med. 1962;87:171–82.
Dorn R, Kopp J, Vogt H, Heidenreich P, Carroll RG, Gulec SA. Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. J Nucl Med. 2003;44(3):451–6.
Van Nostrand D, Atkins F, Moreau S, Aiken M, Kulkarni K, Wu JS, et al. Utility of the radioiodine whole-body retention at 48 hours for modifying empiric activity of 131-iodine for the treatment of metastatic well-differentiated thyroid carcinoma. Thyroid. 2009;19(10):1093–8.
Thomas SR, Maxon HR, Kereiakes JG. In vivo quantitation of lesion radioactivity using external counting methods. Med Phys. 1976;3(4):253–5.
Benua RS. A method and rationale for treating metastatic thyroid carcinoma with the largest safe dose of I-131. Front Thyroidol. 1986, pp. 1317–1321.
Lassmann M, Hänscheid H, Verburg F, Luster M. The use of dosimetry in the treatment of differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2011;55(2):107–15.
Hobbs RF, Wahl RL, Lodge MA, Javadi MS, Cho SY, Chien DT, et al. 124I PET-based 3D-RD dosimetry for a pediatric thyroid cancer patient: real-time treatment planning and methodologic comparison. J Nucl Med. 2009;50(11):1844–7.
Dewaraja YK, Schipper MJ, Roberson PL, Wilderman SJ, Amro H, Regan DD, et al. 131I-tositumomab radioimmunotherapy: Initial tumor dose–response results using 3-dimensional dosimetry including radiobiologic modeling. J Nucl Med. 2010;51(7):1155–62.
Maxon HR, Thomas SR, Hertzberg VS, Kereiakes JG, Chen I-W, Sperling MI, et al. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. New Engl J Med. 1983;309(16):937–41.
Jentzen W, Hoppenbrouwers J, van Leeuwen P, van der Velden D, van de Kolk R, Poeppel TD, et al. Assessment of lesion response in the initial radioiodine treatment of differentiated thyroid cancer using 124I PET imaging. J Nucl Med. 2014;55(11):1759–65.
Wierts R, Brans B, Havekes B, Kemerink GJ, Halders SG, Schaper NN, et al. Dose–response relationship in differentiated thyroid cancer patients undergoing radioiodine treatment assessed by means of 124I PET/CT. J Nucl Med. 2016;57(7):1027–32.
Cristy M, Eckerman K. Specific absorbed fractions of energy at various ages from internal photon sources: 1, methods. Oak Ridge: Oak Ridge National Lab; 1987.
Prideaux AR, Song H, Hobbs RF, He B, Frey EC, Ladenson PW, et al. Three-dimensional radiobiologic dosimetry: Application of radiobiologic modeling to patient-specific 3-dimensional imaging–based internal dosimetry. J Nucl Med. 2007;48(6):1008–16.
Plyku D, Hobbs RF, Huang K, Atkins F, Garcia C, Sgouros G, et al. Recombinant human thyroid-stimulating hormone versus thyroid hormone withdrawal in 124I PET/CT–based dosimetry for 131I therapy of metastatic differentiated thyroid cancer. J Nucl Med. 2017;58(7):1146–54.
Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no 21: a generalized schema for radiopharmaceutical dosimetry: standardization of nomenclature. J Nucl Med. 2009;50(3):477–84.
Plyku D, Lodge M, Van Nostrand D, Sgouros G, Hobbs R. Small volume activity quantification method for absorbed dose calculations. J Nucl Med. 2019;60(supplement 1):1135.
Hobbs R, Jentzen W, Bockisch A, Sgouros G. Monte Carlo-based 3-dimensional dosimetry of salivary glands in radioiodine treatment of differentiated thyroid cancer estimated using 124I PET. Q J Nucl Med Mol Imaging. 2013;57(1):79.
Hobbs RF, Sgouros G. Calculation of the biological effective dose for piecewise defined dose-rate fits. Med Phys. 2009;36(3):904–7.
Hobbs RF, Wahl RL, Frey EC, Kasamon Y, Song H, Huang P, et al. Radiobiologic optimization of combination radiopharmaceutical therapy applied to myeloablative treatment of non-Hodgkin lymphoma. J Nucl Med. 2013;54(9):1535–42.
Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. B J Radiol. 1985;58(690):515–28.
Baechler S, Hobbs RF, Prideaux AR, Wahl RL, Sgouros G. Extension of the biological effective dose to the MIRD schema and possible implications in radionuclide therapy dosimetry. Med Phys. 2008;35(3):1123–34.
Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. B J Radiol. 1989;62(740):679–94.
Millar WT. Application of the linear-quadratic model with incomplete repair to radionuclide directed therapy. Br J Radiol. 1991;64(759):242–51.
Brenner DJ, Hlatky L, Hahnfeldt P, Huang Y, Sachs R. The linear-quadratic model and most other common radiobiological models result in similar predictions of time-dose relationships. Radiat Res. 1998;150(1):83–91.
Van Leeuwen C, Oei A, Crezee J, Bel A, Franken N, Stalpers L, et al. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Rad Oncol. 2018;13(1):1–11.
Gaussen A, Legal J-D, Beron-Gaillard N, Laplanche A, Travagli J-P, Caillou B, et al. Radiosensitivity of human normal and tumoral thyroid cells using fluorescence in situ hybridization and clonogenic survival assay. Int J Radiat Oncol Biol Phys. 1999;44(3):683–91.
Challeton C, Branea F, Schlumberger M, Gaillard N, de Vathaire F, Badie C, et al. Characterization and radiosensitivity at high or low dose rate of four cell lines derived from human thyroid tumors. Int J Radiat Oncol Biol Phys. 1997;37(1):163–9.
Bodey R, Flux G, Evans P. Combining dosimetry for targeted radionuclide and external beam therapies using the biologically effective dose. Cancer Biother Radiopharm. 2003;18(1):89–97.
Sgouros G, Hobbs RF, Atkins FB, Van Nostrand D, Ladenson PW, Wahl RL. Three-dimensional radiobiological dosimetry (3D-RD) with 124 I PET for 131 I therapy of thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38(1):41–7.
Agostinelli S, Allison J, Ka A, Apostolakis J, Araujo H, Arce P, et al. GEANT4—a simulation toolkit. Nucl Instrum Methods Phys Res Sect A. 2003;506(3):250–303.
Chalian H, Töre HG, Horowitz JM, Salem R, Miller FH, Yaghmai V. Radiologic assessment of response to therapy: comparison of RECIST versions 1.1 and 1.0. Radiographics. 2011;31(7):2093–105.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Van Nostrand D, Hobbs R, Atkins FB, Sgouros G. 124 I in differentiated thyroid cancer. In: Wartofsky L, Van Nostrand D, editors. Thyroid cancer: a comprehensive guide to clinical management. Berlin: Springer; 2016. p. 973–89.
Freudenberg LS, Jentzen W, Petrich T, Frömke C, Marlowe RJ, Heusner T, et al. Lesion dose in differentiated thyroid carcinoma metastases after rhTSH or thyroid hormone withdrawal: 124 I PET/CT dosimetric comparisons. Eur J Nucl Med Mol Imaging. 2010;37(12):2267–76.
Khorjekar GR, Van Nostrand D, Garcia C, O’Neil J, Moreau S, Atkins FB, et al. Do negative 124I pretherapy positron emission tomography scans in patients with elevated serum thyroglobulin levels predict negative 131I posttherapy scans? Thyroid. 2014;24(9):1394–9.
Senthamizhchelvan S, Hobbs RF, Song H, Frey EC, Zhang Z, Armour E, et al. Tumor dosimetry and response for 153Sm-ethylenediamine tetramethylene phosphonic acid therapy of high-risk osteosarcoma. J Nucl Med. 2012;53(2):215–24.
Maxon HR 3rd, Englaro EE, Thomas SR, Hertzberg VS, Hinnefeld JD, Chen LS, Smith H, Cummings D, Aden MD. Radioiodine-131 therapy for well-differentiated thyroid cancer—a quantitative radiation dosimetric approach:outcome and validation in 85 patients. J Nucl Med. 1992;33(6):1132–6.
Gear JI, Cox MG, Gustafsson J, Gleisner KS, Murray I, Glatting G, et al. EANM practical guidance on uncertainty analysis for molecular radiotherapy absorbed dose calculations. Eur J Nucl Med Mol Imaging. 2018;45(13):2456–74.
Van Nostrand D. Radioiodine refractory differentiated thyroid cancer: time to update the classifications. Thyroid. 2018;28(9):1083–93.
Funding
This project was supported in part by The Catherine Heron and Al Schneider Fellowship in Thyroid Cancer. Also, we would like to thank the support of donations from our grateful patients at MedStar Washington Hospital Center.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Douglas Van Nostrand: speaker and consultant for Jubilant DraxImage. No competing financial interests exist for the remaining authors.
Ethical approval
The original prospective study forming the source of this analysis of the data presented herein as well as this study itself were approved by the MedStar Health Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Plyku, D., Hobbs, R.F., Wu, D. et al. I-124 PET/CT image-based dosimetry in patients with differentiated thyroid cancer treated with I-131: correlation of patient-specific lesional dosimetry to treatment response. Ann Nucl Med 36, 213–223 (2022). https://doi.org/10.1007/s12149-021-01655-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01655-y