Abstract
Background
Currently, there is a lack of affordable and accessible indicators that can accurately predict immune-related adverse events (irAEs) resulting from the use of immune checkpoint inhibitors (ICIs). In order to address this knowledge gap, our study explore the potential predictive value of two ratios, namely the neutrophil–lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), for irAEs in cancer patients.
Methods
A systematic search was performed in PubMed, Embase, and the Cochrane library. Studies involving NLR or PLR with irAEs were included. Quality and risk of bias of the selected studies were assessed. Forest plots were created based on Cox model analysis. Random effects meta-analyses were conducted to estimate odds ratio (OR) and its 95% confidence interval (CI).
Results
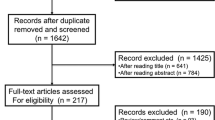
After screening 594 studies, a total of 7 eligible studies with 1068 cancer patients were included. Analysis based on Cox regression showed that low neutrophil–lymphocyte ratio (L-NLR) (OR = 3.02, 95% CI 1.51 to 6.05, P = 0.002) and low platelet-lymphocyte ratio (L-PLR) (OR = 1.83, 95% CI 1.21 to 2.76, P = 0.004) were associated with irAEs. In the subgroup analysis of cut-off value, when the NLR cut-off value was 3, irAEs was significantly correlated with NLR (OR = 2.63, 95% CI 1.63 to 4.26, P < 0.001).
Conclusions
Both L-NLR and L-PLR have been found to be significantly associated with irAEs. Consequently, patients identified as being at a higher risk for irAEs should be subjected to more diligent monitoring and close observation.





Similar content being viewed by others
Data availability
Data will be shared upon reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- H-NLR:
-
High neutrophil–lymphocyte ratio
- H-PLR:
-
High platelet-lymphocyte ratio
- ICIs:
-
Immune checkpoint inhibitors
- irAEs:
-
Immune-related adverse events
- L-NLR:
-
Low neutrophil–lymphocyte ratio
- L-PLR:
-
Low platelet-lymphocyte ratio
- NLR:
-
Neutrophil–lymphocyte ratio
- NOS:
-
Newcastle–Ottawa Scale
- NSCLC:
-
Non-small cell lung cancer
- OR:
-
Odds ratio
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed cell death ligand 1
- PLR:
-
Platelet-lymphocyte ratio
References
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Antonia SJ, Borghaei H, Ramalingam SS, Horn L, De Castro CJ, Pluzanski A, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20(10):1395–408.
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–56.
Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—a systematic review and meta-analysis. Cancer Treat Rev. 2021;92: 102134.
Suazo-Zepeda E, Bokern M, Vinke PC, Hiltermann TJN, de Bock GH, Sidorenkov G. Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Immunol, Immunother: CII. 2021;70(11):3069–80.
von Itzstein MS, Khan S, Gerber DE. Investigational biomarkers for checkpoint inhibitor immune-related adverse event prediction and diagnosis. Clin Chem. 2020;66(6):779–93.
Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18(1):360.
Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36(8):841–8.
Huang H, Zhang H, Onuma AE, Tsung A. Neutrophil elastase and neutrophil extracellular traps in the tumor microenvironment. Adv Exp Med Biol. 2020;1263:13–23.
Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16(1):137.
Jiang L, Luan Y, Miao X, Sun C, Li K, Huang Z, et al. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF-integrin cooperative signalling. Br J Cancer. 2017;117(5):695–703.
Menter DG, Kopetz S, Hawk E, Sood AK, Loree JM, Gresele P, et al. Platelet “first responders” in wound response, cancer, and metastasis. Cancer Metastasis Rev. 2017;36(2):199–213.
Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59.
Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (Amsterdam, Netherlands). 2017;111:176–81.
Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–94.
Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125(1):127–34.
Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e893–900.
Fujimoto A, Toyokawa G, Koutake Y, Kimura S, Kawamata Y, Fukuishi K, et al. Association between pretreatment neutrophil-to-lymphocyte ratio and immune-related adverse events due to immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac Cancer. 2021;12(15):2198–204.
Dharmapuri S, Ozbek U, Saeed A, Muzaffar M, Amara S, Personeni N, et al. Relationship between systemic inflammatory response markers and immune treatment related toxicity (IrAEs) in hepatocellular carcinoma (HCC). J Clin Oncol. 2022;40(16):e16204.
Zhou JG, Wong AHH, Wang H, Tan F, Chen X, Jin SH, et al. Elucidation of the application of blood test biomarkers to predict immune-related adverse events in atezolizumab-treated NSCLC patients using machine learning methods. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.862752.
Ksienski D, Wai ES, Alex D, Croteau NS, Freeman AT, Chan A, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for advanced non-small cell lung cancer patients with high PD-L1 tumor expression receiving pembrolizumab. Transl Lung Cancer Res. 2021;10(1):355–67.
Zhao L, Li Y, Jiang N, Song X, Xu J, Zhu X, et al. Association of blood biochemical indexes and antibiotic exposure with severe immune-related adverse events in patients with advanced cancers receiving PD-1 inhibitors. J Immunother. 2022;45(4):210–6.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45.
Fan X, Wang D, Zhang W, Liu J, Liu C, Li Q, et al. Inflammatory markers predict survival in patients with advanced gastric and colorectal cancers receiving anti-PD-1 therapy. Front Cell Develop Biol. 2021;9: 638312.
Ma Y, Ma X, Wang J, Wu S, Wang J, Cao B. Absolute eosinophil count may be an optimal peripheral blood marker to identify the risk of immune-related adverse events in advanced malignant tumors treated with PD-1/PD-L1 inhibitors: a retrospective analysis. World J Surg Oncol. 2022;20(1):242.
Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist. 2019;24(8):1128–36.
Gannichida A, Nakazawa Y, Kageyama A, Utsumi H, Kuwano K, Kawakubo T. Necessity of neutrophil-to-lymphocyte ratio monitoring for hypothyroidism using nivolumab in patients with cancer. World J Clin Oncol. 2022;13(7):641–51.
Eun Y, Kim IY, Sun JM, Lee J, Cha HS, Koh EM, et al. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep. 2019;9(1):14039.
Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol, Immunother: CII. 2020;69(9):1813–22.
Nakaya A, Kurata T, Yoshioka H, Takeyasu Y, Niki M, Kibata K, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23(4):634–40.
Fukui T, Okuma Y, Nakahara Y, Otani S, Igawa S, Katagiri M, et al. Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: a prospective observational study. Clin Lung Cancer. 2019;20(3):208-14.e2.
Lin X, Deng H, Yang Y, Wu J, Qiu G, Li S, et al. Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor-related pneumonitis in patients with lung cancer. Front Oncol. 2021;11: 698832.
Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17(8):504–15.
Sekine K, Kanda S, Goto Y, Horinouchi H, Fujiwara Y, Yamamoto N, et al. Change in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2018;124:179–88.
Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, Manne A. Risk Factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol. 2022;13: 779691.
Esteban-Fabró R, Willoughby CE, Piqué-Gili M, Montironi C, Abril-Fornaguera J, Peix J, et al. Cabozantinib enhances anti-PD1 activity and elicits a neutrophil-based immune response in hepatocellular carcinoma. Clin Cancer Res. 2022;28(11):2449–60.
Xie W, Hu N, Cao L. Immune thrombocytopenia induced by immune checkpoint inhibitrs in lung cancer: case report and literature review. Front Immunol. 2021;12: 790051.
Ruste V, Goldschmidt V, Laparra A, Messayke S, Danlos FX, Romano-Martin P, et al. The determinants of very severe immune-related adverse events associated with immune checkpoint inhibitors: a prospective study of the French REISAMIC registry. Eur J Cancer. 2021;158:217–24.
Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211.
Lu X, Wan J, Shi H. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios are associated with the efficacy of immunotherapy in stage III/IV non-small cell lung cancer. Oncol Lett. 2022;24(2):266.
Drobni ZD, Zafar A, Zubiri L, Zlotoff DA, Alvi RM, Lee C, et al. Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor–associated myocarditis. J Am Heart Assoc. 2020. https://doi.org/10.1161/JAHA.120.018306.
Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–72.
Michailidou D, Khaki AR, Morelli MP, Diamantopoulos L, Singh N, Grivas P. Association of blood biomarkers and autoimmunity with immune related adverse events in patients with cancer treated with immune checkpoint inhibitors. Sci Rep. 2021;11(1):9029.
Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474–88.
Lee PY, Oen KQX, Lim GRS, Hartono JL, Muthiah M, Huang DQ, et al. Neutrophil-to-lymphocyte ratio predicts development of immune-related adverse events and outcomes from immune checkpoint blockade: a case-control study. Cancers. 2021;13(6):1308.
Funding
This study was supported by grants from Zhejiang Provincial Nature Fund Key Project (LZ23H160007) and Zhejiang Provincial Medical and Pharmaceutical Health Key Project (WKJ-ZJ-2325).
Author information
Authors and Affiliations
Contributions
H-RL and P-FZ: contributed equally to this work; Z-LC, and LY: revised the manuscript. All authors wrote and revised the manuscript and issued the final approval for the version to be submitted.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Ethical Approval is not applicable for this article.
Patient consent
Patient consent was not required as this was a systematic review and meta‐analysis of published articles.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12094_2023_3313_MOESM1_ESM.xlsx
Supplementary file1 Supplementary Table 1 Grade and subtype of occurrence of irAEs in the included literature. (XLSX 15 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, HR., Zhu, PF., Deng, YY. et al. Predictive value of NLR and PLR for immune-related adverse events: a systematic review and meta-analysis. Clin Transl Oncol 26, 1106–1116 (2024). https://doi.org/10.1007/s12094-023-03313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03313-3