Abstract
Natural products offer promising potential for the development of new therapies for Alzheimer's disease (AD). Blackberry fruits are rich in phytochemical compounds capable of modulating pathways involved in neuroprotection. Additionally, drug repurposing and repositioning could also accelerate the development of news treatments for AD. In light of the reduced brain glucose metabolism in AD, an alternative approach has been the use of the drug metformin. Thus, the aim of this study was to evaluate the effect of treatment with blackberry extract in a model of AD induced by streptozotocin (STZ) and compare it with metformin treatment. Male rats were divided into groups: I - Control; II - STZ; III - STZ + blackberry extract (100 mg/kg); IV - STZ + blackberry extract (200 mg/kg) and V - STZ + metformin (150 mg/kg). The animals received intracerebroventricular injection of STZ or buffer. Seven days after the surgical procedure, the animals were treated orally with blackberry extract or metformin for 21 days. Blackberry extract and metformin prevented the memory impairment induced by STZ. In animals of group II, an increase in acetylcholinesterase activity, phosphorylated tau protein, IL-6, oxidative damage, and gene expression of GSK-3β and Nrf2 was observed in the hippocampus. STZ induced a decrease in IL-10 levels and down-regulated the gene expression of Akt1, IRS-1 and FOXO3a. Blackberry extract and metformin prevented the alterations in acetylcholinesterase activity, IL-6, GSK3β, Nrf2, and oxidative damage. In conclusion, blackberry extract exhibits multi-target actions in a model of AD, suggesting new therapeutic potentials for this neurodegenerative disease.
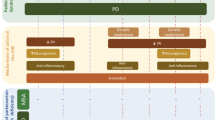
Graphical Abstract






Similar content being viewed by others
Data Availability
Please contact the authors for data request.
References
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A et al (2009) Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 396:413–446. https://doi.org/10.1016/S0140-6736(20)30367
Breijyeh Z, Karaman R (2020) Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules 25:5789. https://doi.org/10.3390/molecules25245789
Zhang XX, Tian Y, Wang ZT, Ma YH, Tan L, Yu JT (2021) The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. J Prev Alzheimers Dis 8:313–321. https://doi.org/10.14283/jpad.2021.15
Zhang H, Wei W, Zhao M, Ma L, Jiang X, Pei H, Cao Y, Li H (2021) Interaction between Aβ and Tau in the pathogenesis of Alzheimer’s disease. Int J Biol Sci 17:2181–2192. https://doi.org/10.7150/ijbs.57078
Bai R, Guo J, Ye XY, Xie Y, Xie T (2022) Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev 77:101619. https://doi.org/10.1016/j.arr.2022.101619
Leng F, Edison P (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17:157–172. https://doi.org/10.1038/s41582-020-00435-y
An Y, Varma VR, Varma S, Casanova R, Dammer E, Pletnikova O, Chia CW, Egan JM et al. (2018) Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement 14:318–329. https://doi.org/10.1016/j.jalz.2017.09.011
Sharma K (2019) Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol Med Rep 20:1479–1487. https://doi.org/10.3892/mmr.2019.10374
Beshir SA, Aadithsoorya AM, Parveen A, Goh SSL, Hussain N, Menon VB (2022) Aducanumab therapy to treat Alzheimer’s disease: a narrative review. Alzheimer’s Dis 9:9343514. https://doi.org/10.1155/2022/9343514
Michailidis M, Tata DA, Moraitou D, Kavvadas D, Karachrysafi S, Papamitsou T, Vareltzis P, Papaliagkas V (2022) Antidiabetic drugs in the treatment of Alzheimer’s disease. Int J Mol Sci 23:4641. https://doi.org/10.3390/ijms23094641
Koo BK, Kim LK, Lee JY, Moon MK (2019) Taking metformin and cognitive function change in older patients with diabetes. Geriatr Gerontol Int 19:755–761. https://doi.org/10.1111/ggi.13692
Pomilio C, Pérez NG, Calandri I, Crivelli L, Allegri R, Sevlever G, Saravia F (2022) Diabetic patients treated with metformin during early stages of Alzheimer’s disease show a better integral performance: data from ADNI study. Geroscience 44:1791–1805. https://doi.org/10.1007/s11357-022-00568-6
Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z, Huang J, Xu B et al (2018) Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 69:351–363. https://doi.org/10.1016/j.bbi.2017.12.009
Nassar SZ, Badae NM, Issa YA (2018) Effect of amylin on memory and central insulin resistance in a rat model of Alzheimer’s disease. Arch Physiol Biochem 126:326–334. https://doi.org/10.1080/13813455.2018.1534244
Pilipenko V, Narbute K, Pupure J, Langrate IK, Muceniece R, Kluša V (2020) Neuroprotective potential of antihyperglycemic drug metformin in streptozocininduced rat model of sporadic Alzheimer’s disease. Eur J Pharmacol 881:173290. https://doi.org/10.1016/j.ejphar.2020.173290
Saffari PM, Alijanpour S, Takzaree N, Sahebgharani M, Etemad-Moghadam S, Noorbakhsh F, Partoazar A (2020) Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin induced Alzheimer’s disease model. Life Sci 225:117861. https://doi.org/10.1016/j.lfs.2020.117861
Gutierres JM, Carvalho FB, Schetinger PC, Marisco P, Agostinho P, Rodrigues M, Rubin MA, Schmatz R et al (2014) Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci 96:7–17. https://doi.org/10.1016/j.lfs.2013.11.014
Pacheco SM, Soares MSP, Gutierres JM, Gerzon MFB, Carvalho FB, Azambuja JH, Schetinger MRC, Stefanello FM et al (2018) Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J Nutr Biochem 56:193–204. https://doi.org/10.1016/j.jnutbio.2018.02.014
Chaves V, Soares MS, Spohr L, Teixeira L, Vieira A, Constantino L, Dal Pizzol F, Lencina C et al (2020) Blackberry extract improves behavioral and neurochemical dysfunctions in a ketamine induced rats model of mania. Neurosci Lett 714:134566. https://doi.org/10.1016/j.jnutbio.2018.02.014
Soares MSP, Luduvico KPL, Chaves VC, Spohr L, Meine BM, Lencina CL, Reginatto FH, Spanevello RM et al (2021) The protective action of Rubus sp. fruit extract against oxidative damage in mice exposed to lipopolysaccharide. Neurochem Res 5:1129–1140. https://doi.org/10.1007/s11064-021-03248-7
De Mello JE, Luduvico KP, Santos A, Teixeira FC, Cardoso JS, Aguiar MSS, Cunico W, Vizzotto M et al (2023) Therapeutic potential of blackberry extract in the preventing memory deficits and neurochemical alterations in the cerebral cortex, hippocampus and cerebellum of a rat model with amnesia. Metab Brain Dis. https://doi.org/10.1007/s11011-023-01175-w
Shonesya BC, Thiruchelvama K, Parameshwarana K, Rahmana EA, Karuppagoundera SS, Hugginsb KW, Pinkertc CA, Amina R et al (2012) Central insulin resistance and synaptic dysfunction in intracerebroventricular-streptozotocin injected rodents. Neurobiol Aging 33:5–18. https://doi.org/10.1016/j.neurobiolaging.2010.12.002
Knezovic A, Osmanovic-Barilar J, Curlin M, Hof PR, Simic G, Riederer P, Salkovic-Petrisic M (2015) Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer’s disease. J Neural Transm 122:577–592. https://doi.org/10.1007/s00702-015-1394-4
Teixeira FC, Gutierres JM, Soares MSP, Mattos B, Spohr L, Couto CAT, Bona N, Assmann C et al (2020) Inosine protects against impairment of memory induced by experimental model of Alzheimer disease: a nucleoside with multitarget brain actions. Psychopharmacol 237:811–823. https://doi.org/10.1007/s00213-019-05419-5
Ellman G, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ali SF, Lebel CP, Bondy SC (1992) Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicol 13:637–648
Esterbauer H, Cheeseman K (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Huang WC, Lin YS, Wang CY, Tsai CC, Tseng HC, Chen CL, Lu PJ, Chen PS et al. (2009) Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Mol Neurobiol 54:6507–6522. https://doi.org/10.1111/j.1365-2567.2008.02959.x
Aksenov MY, Markesbery WR (2021) Change in thiol content and expression of glutathione redox system gene in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein– dye binding. Anal Biochem 72:248–254
Furman BL (2021) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc 4:e78. https://doi.org/10.1002/cpz1.78
Guo Z, Chen Y, Mao YF, Zheng T, Jiang Y, Yan Y (2017) Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci Rep 7:45971. https://doi.org/10.1038/srep45971
Opitz B (2014) Memory function and the hippocampus. Front Neurol Neurosci 34:51–59. https://doi.org/10.1159/000356422
Bassani TB, Bonato JM, Machado MMF, Cóppola-Segovia V, Moura ELR, Zanata SM, Oliveira RMMW, Vital MABF (2018) Decrease in adult neurogenesis and neuroinflammation are involved in spatial memory impairment in the streptozotocin-induced model of sporadic Alzheimer’s disease in rats. Mol Neurobiol 55:4280–4296. https://doi.org/10.1007/s12035-017-0645-9
Rao YL, Ganaraja B, Murlimanju BV, Joy T, Krishnamurthy A, Agrawal A (2022) Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech 2:55. https://doi.org/10.1007/s13205-022-03123-4
Kazkayasi I, Telli G, Nemutlu E, Uma S (2022) Intranasal metformin treatment ameliorates cognitive functions via insulin signaling pathway in ICV-STZ-induced mice model of Alzheimer’s disease. Life Sci 299:120538. https://doi.org/10.1016/j.lfs.2022.120538
Ferreira-Vieira T, Guimarães I, Silva F, Ribeiro F (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14:101–115. https://doi.org/10.2174/1570159x13666150716165726
Soreq H, Seidman S (2002) Acetylcholinesterase — new roles for an old actor. Nat Rev Neurosci 2:294–302. https://doi.org/10.1038/35067589
Piovesana R, Salazar Intriago MS, Dini L, Tata AM (2021) Cholinergic modulation of neuroinflammation: focus on α7 nicotinic receptor. Int J Mol Sci 22:4912. https://doi.org/10.3390/ijms22094912
Aksoz E, Gocmez SS, Sahin TD, Aksit D, Aksit H, Utkan T (2019) The protective effect of metformin in scopolamine-induced learning and memory impairment in rats. Pharmacol Rep 71:818–825. https://doi.org/10.1016/j.pharep.2019.04.015
Grieb P (2016) Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol Neurobiol 53:1741–1752. https://doi.org/10.1007/s12035-015-9132-3
Xiong R, Wang X, Wu J, Tang Y, Qiu W, Shen X, Teng J, Pan R et al (2020) Polyphenols isolated from lychee seed inhibit Alzheimer’s disease-associated Tau through improving insulin resistance via the IRS-1/PI3K/Akt/GSK-3β pathway. J Ethnopharmacol 251:112548. https://doi.org/10.1016/j.jep.2020.112548
Revett TJ, Baker GB, Jhamandas J, Kar S (2013) Glutamate system, amyloid (beta) peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J Psych Neurosci 38:6–23. https://doi.org/10.1503/jpn.110190
Qin L, Zhang J, Qin M (2013) Protective effect of cyanidin 3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats. Neurosci Lett 534:285–288. https://doi.org/10.1016/j.neulet.2012.12.023
De Oliveira AC, Candelario-Jalil E, Fiebich BL, Santos Mda S, Palotás A, dos Reis HJ (2015) Neuroinflammation and neurodegeneration: pinpointing pathological and pharmacological targets. Biomed Res Int 2015:487241. https://doi.org/10.1016/10.1155/2015/487241
Amin FU, Shah SA, Badshah H, Khan M, Kim MO (2017) Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1–42-induced oxidative stress. J Nanobiotechnol 15:12.https://doi.org/10.1186/s12951-01
Hammad AM, Ibrahim YA, Khdair SI, Hall FS, Alfaraj M, Jarrar Y, Abed AF (2021) Metformin reduces oxandrolone- induced depression-like behavior in rats via modulating the expression of IL-1β, IL-6, IL-10 and TNF-α. Behav Brain Res 414:113475. https://doi.org/10.1016/j.bbr.2021.113475
Dos Santos A, Teixeira FC, da Silva DS, Veleda TA, de Mello JE, Luduvico KP, Tavares RG, Stefanello FM et al (2023) Thiazolidin-4-one prevents against memory deficits, increase in phosphorylated tau protein, oxidative damage and cholinergic dysfunction in Alzheimer disease model: Comparison with donepezil drug. Brain Res Bull 193:1–10. https://doi.org/10.1016/j.brainresbull.2022.11.015
Demirci-Çekiç S, Özkan G, Avan AN, Uzunboy S, Çapanoğlu E, Apak R (2022) Biomarkers of oxidative stress and antioxidant defense. J Pharm Biomed Anal 209:114477. https://doi.org/10.1016/j.jpba.2021.114477
Forman HJ, Zhang H (2021) Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 20:689–709. https://doi.org/10.1038/s41573-021-00233-1
Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 22:1583. https://doi.org/10.3390/molecules24081583
Davies DA, Adlimoghaddam A, Albensi BC (2021) Role of Nrf2 in synaptic plasticity and memory in Alzheimer’s disease. Cells 10:1884. https://doi.org/10.3390/cells10081884
Nho RS, Hergert P (2014) FoxO3a and disease progression. World J Biol Chem 5:346–354. https://doi.org/10.4331/wjbc.v5.i3.346
Xu S, Zhang X, Ma Y, Chen Y, Xie H, Yu L, Wang J, Xu SQ et al (2022) FOXO3a alleviates the inflammation and oxidative stress via regulating TGF-β and HO-1 in ankylosing spondylitis. Front Immunol 13:935534. https://doi.org/10.3389/fimmu.2022.935534
Bendokas V, Stanys V, Mažeikienė I, Trumbeckaite S, Baniene R, Liobikas J (2020) Anthocyanins: from the field to the antioxidants in the body. Antioxidants (Basel) 9:819. https://doi.org/10.3390/antiox9090819
Borkowski T, Szymusiak H, Gliszczyńska-Rwigło A, Rietjens IM, Tyrakowska B (2005) Radical scavenging capacity of wine anthocyanins is strongly pH-dependent. J Agric Food Chem 53:5526–5534. https://doi.org/10.1021/jf0478556
Giampieri F, Alvarez-Suarez JM, Mazzoni L, Forbes-Hernandez TY, Gasparrini M, Gonzàlez-Paramàs AM, Santos-Buelga C, Quiles JL et al (2014) Polyphenol-rich strawberry extract protects human dermal fibroblasts against hydrogen peroxide oxidative damage and improves mitochondrial functionality. Molecules 19:7798-816.https://doi.org/10.3390/molecules19067798
Zhang B, Buya M, Qin W, Sun C, Cai H, Xie Q, Xu B, Wu Y (2013) Anthocyanins from Chinese bayberry extract activate transcription factor Nrf2 in β cells and negatively regulate oxidative stress-induced autophagy. J Agric Food Chem 61:8765–8772. https://doi.org/10.1021/jf4012399
Chukwunonso Obi B, Chinwuba Okoye T, Okpashi VE, Nonye Igwe C, Olisah Alumanah E (2016) Comparative study of the antioxidant effects of metformin, glibenclamide, and repaglinide in alloxan-induced diabetic rats. J Diabetes Res 2016:1635361. https://doi.org/10.1155/2016/1635361
Lu XY, Huang S, Chen QB, Zhang D, Li W, Ao R, Leung FC, Zhang Z et al (2020) Metformin Ameliorates Aβ Pathology by insulin-degrading enzyme in a transgenic mouse model of Alzheimer’s Dis. Oxid Med Cell Longev 19:2020:2315106. https://doi.org/10.1155/2020/2315106
Teleanu DM, Niculescu AG, Lungu II, Radu CI, Vladâcenco O, Roza E, Costăchescu B, Grumezescu AM et al (2022) An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci. 25:5938. https://doi.org/10.3390/ijms23115938
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20:148–160. https://doi.org/10.1038/s41583-019-0132-6
Acknowledgements
The authors would like to acknowledge the Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA - Clima Temperado) in Pelotas, RS, Brazil, for providing blackberry fruits.
Funding
This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS). This study was financed, in part, by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES)—Finance code 001. R.M.S is a recipient of the CNPq fellowship (310472/2021–0).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparations, data collection and analysis were performed by J.E.M.; F.C.T.; A.S.; K.L.; M.S.S.A.; W.B.; V.C.F.; R.T.; A.S. The first draft of the manuscript was written by J.E.M and R.M.S. Review, editing and funding acquisition was performed by F.M.S. and R.M.S. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
All animal experiments were carried out in accordance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals and approved by the Ethics Committee on the Use of Animals of the Federal University of Pelotas (protocol number: protocol number: 42067–2019). All animals received care in compliance with the principles of laboratory animal standards.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Mello, J.E., Teixeira, F.C., dos Santos, A. et al. Treatment with Blackberry Extract and Metformin in Sporadic Alzheimer’s Disease Model: Impact on Memory, Inflammation, Redox Status, Phosphorylated Tau Protein and Insulin Signaling. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04062-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04062-2