Abstract
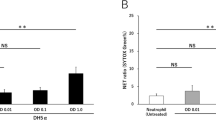
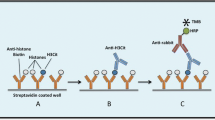
Activated neutrophils release neutrophil extracellular traps (NETs) composed of chromatin filaments containing bactericidal proteins and enzymes. This process, known as NETosis, is an innate host defense mechanism. However, NET accumulation can lead to uncontrolled inflammation and organ damage. Therefore, NET detection provides clinically important information for the assessment of inflammatory conditions. We investigated whether quantification of citrullinated fibrinogen (C-Fbg), which is catalyzed by peptidylarginine deiminase (PAD) released during NETosis, can be used to detect NETs. Human neutrophils were stimulated with fibrinogen using phorbol 12-myristate 13-acetate (PMA). The myeloperoxidase (MPO)-DNA complex and C-Fbg concentrations in the culture supernatants were quantified using an enzyme-linked immunosorbent assay. The protein levels of peptidylarginine deiminase 2 and 4 in culture supernatants and mRNA levels in PMA-stimulated neutrophils were also assessed. The levels of the MPO-DNA complex in the supernatants of PMA-stimulated neutrophils increased, indicating NETosis. C-Fbg level also increased, which was suppressed by both NETosis and PAD inhibitors. PAD2 was detected in the culture supernatant; however, PAD4, but not PAD2, mRNA levels increased in PMA-stimulated neutrophils. This study quantitatively demonstrates that fibrinogen is citrullinated by PAD derived from PMA-stimulated neutrophils upon NETosis. Although further studies are needed for clinical application, quantification of C-Fbg in blood may help detect the presence of NETs.






Similar content being viewed by others
Data Availability
Data will be made available by the corresponding author upon reasonable request.
References
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75.
Mutua V, Gershwin LJ. A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin Rev Allergy Immunol. 2021;61(2):194–211.
Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE. 2007;379:pe11.
Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol. 2020;11:1749.
Moschonas IC, Tselepis AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. 2019;288:9–16.
Bonaventura A, Vecchié A, Abbate A, Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells. 2020;9:1.
Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21(7):815–9.
Lee KH, Kronbichler A, Park DD, Park Y, Moon H, Kim H, et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev. 2017;16(11):1160–73.
Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–8.
Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–8.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5.
Spengler J, Lugonja B, Ytterberg AJ, Zubarev RA, Creese AJ, Pearson MJ, et al. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. 2015;67(12):3135–45.
Masuda S, Nakazawa D, Shida H, Miyoshi A, Kusunoki Y, Tomaru U, et al. NETosis markers: quest for specific, objective, and quantitative markers. Clin Chim Acta. 2016;459:89–93.
Thålin C, Aguilera K, Hall NW, Marunde MR, Burg JM, Rosell A, et al. Quantification of citrullinated histones: development of an improved assay to reliably quantify nucleosomal H3Cit in human plasma. J Thromb Haemost. 2020;18(10):2732–43.
Bronkhorst AJ, Aucamp J, Pretorius PJ. Cell-free DNA: preanalytical variables. Clin Chim Acta. 2015;450:243–53.
Hayden H, Ibrahim N, Klopf J, Zagrapan B, Mauracher LM, Hell L, et al. ELISA detection of MPO-DNA complexes in human plasma is error-prone and yields limited information on neutrophil extracellular traps formed in vivo. PLoS ONE. 2021;16(4):e0250265.
Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–20.
Doolittle RF. Fibrinogen and fibrin. Sci Am. 1981;245(6):126–35.
Nakayama-Hamada M, Suzuki A, Kubota K, Takazawa T, Ohsaka M, Kawaida R, et al. Comparison of enzymatic properties between hPADI2 and hPADI4. Biochem Biophys Res Commun. 2005;327(1):192–200.
Darrah E, Rosen A, Giles JT, Andrade F. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann Rheum. 2012;71(1):92–8.
Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden BT. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18(4):581–8.
Mastronardi FG, Wood DD, Mei J, Raijmakers R, Tseveleki V, Dosch HM, et al. Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor–induced peptidylarginine deiminase 4 translocation. J Neurosci. 2006;26(44):11387–96.
Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133(20):2178–85.
Blachère NE, Parveen S, Fak J, Frank MO, Orange DE. Inflammatory but not apoptotic death of granulocytes citrullinates fibrinogen. Arthritis Res Ther. 2015;17:369.
Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40.
Thieblemont N, Wright HL, Edwards SW, Witko-Sarsat V. Human neutrophils in auto-immunity. Semin Immunol. 2016;28(2):159–73.
Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis. 2016;75(10):1866–75.
van de Stadt LA, de Koning MH, van de Stadt RJ, Wolbink G, Dijkmans BA, Hamann D, et al. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum. 2011;63(11):3226–33.
Wu CY, Yang HY, Lai JH. Anti-Citrullinated Protein Antibodies in Patients with Rheumatoid Arthritis: Biological Effects and Mechanisms of Immunopathogenesis. Int J Mol Sci. 2020;21:11.
Muller S, Radic M. Citrullinated autoantigens: from diagnostic markers to pathogenetic mechanisms. Clin Rev Allergy Immunol. 2015;49(2):232–9.
Raijmakers R, van Beers JJ, El-Azzouny M, Visser NF, Božič B, Pruijn GJ, et al. Elevated levels of fibrinogen-derived endogenous citrullinated peptides in synovial fluid of rheumatoid arthritis patients. Arthritis Res Ther. 2012;14(3):R114.
Terasawa F, Higuchi Y, Arai S, Okumura N. Measurement of the serum levels of citrullinated fibrinogen and its antibodies in rheumatoid arthritis patients. J Analytic Bio-Sci. 2016;39:263–70 ((In Japanease)).
Demoruelle MK, Bowers E, Lahey LJ, Sokolove J, Purmalek M, Seto NL, et al. Antibody responses to citrullinated and noncitrullinated antigens in the sputum of subjects with rheumatoid arthritis and subjects at risk for development of rheumatoid arthritis. Arthritis Rheumatol. 2018;70(4):516–27.
Hirota-Kawadobora M, Kani S, Terasawa F, Fujihara N, Yamauchi K, Tozuka M, et al. Functional analysis of recombinant Bbeta15C and Bbeta15A fibrinogens demonstrates that Bbeta15G residue plays important roles in FPB release and in lateral aggregation of protofibrils. J Thromb Haemost. 2005;3(5):983–90.
Okumura N, Haneishi A, Terasawa F. Citrullinated fibrinogen shows defects in FPA and FPB release and fibrin polymerization catalyzed by thrombin. Clin Chim Acta. 2009;401(1–2):119–23.
Fujimura S, Higuchi Y, Usami Y, Yamaura M, Higuchi T, Terasawa F, et al. Changes in serum citrullinated fibrinogen concentration associated with the phase of bacteremia patients. Clin Chim Acta. 2021;512:127–34.
Sil P, Yoo DG, Floyd M, Gingerich A, Rada B. High throughput measurement of extracellular DNA release and quantitative NET formation in human neutrophils in vitro. J Vis Exp. 2016;112:52779.
Yoo DG, Floyd M, Winn M, Moskowitz SM, Rada B. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase–DNA and neutrophil elastase–DNA complexes. Immunol Lett. 2014;160(2):186–94.
Rada B. Neutrophil extracellular traps. Methods Mol Biol. 1982;2019:517–28.
Kenny EF, Herzig A, Krüger R, Muth A, Mondal S, Thompson PR, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6:e24437.
Hawez A, Al-Haidari A, Madhi R, Rahman M, Thorlacius H. MiR-155 Regulates PAD4–dependent formation of neutrophil extracellular traps. Front Immunol. 2019;10:2462.
Causey CP, Jones JE, Slack JL, Kamei D, Jones LE, Subramanian V, et al. The development of N-α-(2-carboxyl)benzoyl-N(5)-(2-fluoro-1-iminoethyl)-l-ornithine amide (o-F-amidine) and N-α-(2-carboxyl)benzoyl-N(5)-(2-chloro-1-iminoethyl)-l-ornithine amide (o-Cl-amidine) as second generation protein arginine deiminase (PAD) inhibitors. J Med Chem. 2011;54(19):6919–35.
Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11(3):189–91.
Czaikoski PG, Mota JM, Nascimento DC, Sônego F, Castanheira FV, Melo PH, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE. 2016;11(2):e0148142.
Zhou Y, Chen B, Mittereder N, Chaerkady R, Strain M, An LL, et al. Spontaneous secretion of the citrullination Enzyme PAD2 and cell surface exposure of PAD4 by neutrophils. Front Immunol. 2017;8:1200.
Zhou Y, An LL, Chaerkady R, Mittereder N, Clarke L, Cohen TS, et al. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci Rep. 2018;8(1):15228.
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41.
Hoppenbrouwers T, Autar ASA, Sultan AR, Abraham TE, van Cappellen WA, Houtsmuller AB, et al. In vitro induction of NETosis: comprehensive live imaging comparison and systematic review. PLoS ONE. 2017;12(5):e0176472.
Zhang Z, Niu R, Zhao L, Wang Y, Liu G. Mechanisms of neutrophil extracellular trap formation and regulation in cancers. Int J Mol Sci. 2023;24(12):10265.
Huang Y, Ding Y, Wang B, Ji Q, Peng C, Tan Q. Neutrophils extracellular traps and ferroptosis in diabetic wounds. Int Wound J. 2023;20(9):3840–54.
Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23(3):279–87.
Acknowledgements
All figures used in this study were originally prepared by the authors and have not been published elsewhere.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant No. JP20K07822).
Author information
Authors and Affiliations
Contributions
Conceptualization: TS, TH, NO, and YH. Writing—original draft: TS, TH, NO, and YH. Investigation: TS, TI, SH, HO, SF, and YH. Data curation—formal analysis: TS, TI, SH, HO, SF, and YH. All the authors have read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Research involving human participants
This study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the Ethical Review Board of Shinshu University School of Medicine (approval number 5342).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sue, T., Ichikawa, T., Hattori, S. et al. Quantitative evaluation of citrullinated fibrinogen for detection of neutrophil extracellular traps. Immunol Res (2023). https://doi.org/10.1007/s12026-023-09446-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12026-023-09446-5