Abstract
Lipocalin 2 (Lcn2), also known as neutrophil gelatinase–associated lipocalin, is an innate immune protein encoded by the LCN2 gene. In this study, we investigated various roles and functions of Lcn2 characterized in a systems-based format and evaluated its therapeutic potentials and clinical relevance for diagnosis and prognosis. An additional systematic presentation was presented for 70 ongoing clinical trials utilizing Lcn2 in the diagnostic and prognostic setting as a key outcome measure. With trials being conducted through December 2030, Lcn2 will become all the more relevant given its associations with diseases as a prognostic biomarker. Data also suggests that it plays a role in pathological conditions. The gaps in our understanding of Lcn2, once filled, may improve the immune mediation of acute and chronic disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
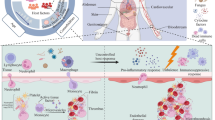
Lipocalin 2 (Lcn2), an innate immune protein also known as neutrophil gelatinase–associated lipocalin (NGAL), is encoded by the LCN2 gene. Lcn2 was initially identified in 1989 by identification of its messenger RNA named 24p3 in simian virus 40 (SV-40)–infected kidney cells of mouse models [1]. It was originally named neutrophil-gelatinase-associated lipocalin (NGAL) as it was first isolated in neutrophil granules of humans [2]. It is a member of the lipocalin protein family that is a group of diverse proteins with a complex β-barrel 3D structure forming an enclosing calyx [3]. The flexible structure also contains a hydrophobic pocket allowing the Lcn2 protein to carry out key functions in cell regulation, proliferation, and differentiation [4]. It is a 25-kDa glycoprotein with key expression in bodily fluids and tissues and is protease-resistant due to its complex formation with matrix metalloprotease-9 (MMP-9) in human neutrophils [5, 6]. The release of Lcn2 is strongly upregulated during inflammation by epithelial cells, and it is also released by epithelial cells, macrophages, hepatocytes, and adipocytes. It transports small hydrophobic molecules such as lipids, steroid hormones, and retinoids [7].
As an organic chelator of iron, Lcn2 limits bacterial growth by sequestering iron-containing siderophores [8, 9]. This promotes the bacteriostatic action potential of Lcn2 by depriving the bacteria of their nutrition and inhibiting their growth [8]. Due to its high affinity to iron, it acts as a regulator of iron-responsive genes [6]. The strong affinity of Lcn2 for iron is also evident in its effects on human proteins including norepinephrine [6]. Previous studies conducted on mice have reported increased susceptibility to bacterial infections following a lack of the LCN2 gene. Other functions of the Lcn2 protein include the moderation of the immune and inflammatory response, induction of apoptosis, and maintenance of skin homeostasis and insulin sensitivity [10,11,12,13]. The Lcn2 protein, measured in blood, urine, and feces, also serves as a significant biomarker during inflammation, ischemia, infection, and acute kidney injury [14, 15]. In this study, we investigated various roles and functions of Lcn2 and evaluated its therapeutic potential and clinical relevance as a diagnostic and prognostic marker.
Methodology
A literature review was conducted to report existing evidence of lipocalin. This was divided into different systems including (i) inflammatory mediation and iron homeostasis, (ii) malignancy, (iii) kidneys, (iv) gastrointestinal tract, (v) biomarker potential, (vi) other potential functions, and (vii) therapeutic potential. To identify and include the studies in the literature review portion, a search was conducted in the PubMed database and the Google Scholar search engine. The keywords used in this study are found in Table 1. The findings were discussed based on the aforementioned systems in a literature-based scoping format.
In the inclusion of ongoing clinical trials of lipocalin 2, a systematic strategy was adopted as recommended by PRISMA Statement 2020 guidelines. A search was conducted on ClinicalTrials.Gov where public and privately funded clinical trials are stored. A search of the World Health Organization’s International Clinical Trials Registry Platform was also conducted until November 20, 2022. A total of 232 records were located, of which 94 were reviewed in full. Of these, 70 records were added to this review, post-reviewed by two mid-career researchers (Z.S. and A.S.). No limitations were made for the gender or age groups of the included participants in the clinical trials. Disagreements of the tabulations during the systematic review inclusion phase were resolved through active consensus. The extraction was led by all researchers. The findings of all records were tabulated under the following headings: NCT number, Title, Current status, Target conditions, Interventions, Outcome measures, Enrollment, Funded by, Study type, Study designs, Completion date, and Locations. During the inclusion phase, the inter-reviewer kappa score was 0.915 suggesting excellent agreement between the reviewers.
Lcn2 as a modulator of inflammation and iron homeostasis
Lcn2 is a critical regulator of iron homeostasis during physiological and inflammatory processes. The role of Lcn2 as an inhibitor of bacterial growth has been well established, and it is able to prevent the bacteria from acquiring iron. Many acute-phase reactants have been identified including C-reactive protein, serum amyloid A, ferritin, and hepcidin, and they have effects on the ongoing inflammatory process. Recently, Lcn2 was identified and is observed to regulate host responses to inflammation by maintaining iron homeostasis. Lcn2 does not bind to iron directly and instead forms a ternary complex with it along with a siderophore serving as a cofactor. Siderophores have a high affinity for insoluble ferric irons and are classified into chemical groups namely catecholates, carboxylates, and hydroxamates. Lcn2 is not able to sequester hydroxamates, but it is able to bind to the other two chemical groups of siderophores [13]. All microbes except Lactobacillus and Borrelia spp. [16] require iron for maintaining growth in their hosts. The role of Lcn2 as a binder of catecholate- and carboxylate-type siderophores promotes nutritional immunity against iron-dependent microbes in humans.
Inflammatory bowel disease
The role of Lcn2 has been observed in the innate immune response, but its mechanisms of action in intestinal inflammation are less known. The underlying pathophysiology of inflammatory bowel disease has been correlated with increased expression of Lcn2, but its role has not been explored until recently [5]. Janas et al. identified the elevation of serum NGAL in children presenting with inflammatory bowel disease (IBD) [5]. Mouse models of inflammatory bowel disease (IBD) have ascertained the role of Lcn2 during intestinal inflammation and gut bacterial dysbiosis. Mouse models were observed for Lcn2 expression in murine colitis models and during microbiota ablation/restoration. Lcn2 was induced during inflammation, and it was due to the presence of gut microbiota and MyD88 signaling [17]. The activation of toll-like receptors (TLRs) is carried out by bacterial products, and it results in the subsequent encoding of genetic products specifically aimed to promote host defenses. The intestine consists of a wide range of commensal bacteria, and the role of TLRs may not be limited to that of an on/off switch. Flagellin receptor TLR5 plays a critical role in protecting the enteric microbiota. Vijay-Kumar et al. (2007) conducted a study where mice lacking TLR5 developed spontaneous colitis, but the subsequent deletion of TLR4 served as a protective mechanism. In instances where both TLR4 and TLR5 were deleted, bacterial loads were high in the colon but there were no elevated laboratory markers or clinical evidence of colitis [18]. The findings suggest the correlation of innate immune deficiency with higher incidences of inflammatory bowel disease [18]. Higher serum Lcn2 levels were also observed in mice with elevated serum amyloid A (SAA), an acute-phase marker representing the degree of intestinal inflammation in both mice and humans [18,19,20].
Malignancy
In the past few decades, Lcn2 has gained prominence for its role as a potential biomarker and modulator of malignancy. Its ability to serve as an intracellular iron carrier and protector of MMP-9 from proteolytic degradation allows it to serve contradictory functions in malignant cells. Elevated Lcn2 levels have been observed in multiple epithelial tumors including ovarian tumors [21]. Lcn2 is also over-expressed in other tumors and functions by inhibiting apoptosis in thyroid cancer–infected cells, modulating invasiveness and angiogenesis in pancreatic cancer cells, and increasing invasiveness in breast and colorectal cancer [22]. Chronic inflammation has been identified as a risk factor for the development of epithelial-derived malignancies (Bower et al., 2005). Clinical oncology is paying close attention to the potential roles of Lcn2 as a diagnostic and prognostic biomarker for early diagnosis and response to treatments [23].
Breast cancer
Lcn2 levels in plasma and urine are consistently elevated in invasive breast cancer. The functions of Lcn2 in breast cancer progression are observed by its actions on mesenchymal markers. The protein elevates vimentin and fibronectin while downregulating the epithelial marker E-cadherin. As a result, the cell motility and invasiveness are increased leading to an epithelial-to-mesenchymal transition (EMT). Contrarily, the silencing of Lcn2 in invasive breast cancer results in reduced invasiveness. Lcn2 also reduces the expression of estrogen receptor (ER) α and increases the expression of Slug, the primary transcription factor responsible for EMT [22]. Inhibition of Lcn2 prevents breast tumorigenesis and invasion as observed in mouse models. Without Lcn2, mice had delayed the formation of mammary tumors and invasion as well as a reduced migration ability of Her2 + cells [24].
Thyroid cancer
NF-κB is activated in tumors of thyroid origin, especially those of anaplastic origin, and blockade of its activity leads to an increased susceptibility of thyroid cancer cells to undergo chemotherapy-induced apoptosis in mice models. Proteomic analysis allowed Lcn2 identification, and it is secreted by the anaplastic thyroid cancer cell line, FRO cells. Owing to Lcn2’s ability to bind to and transport intra-cellular iron, NF-κB works in conjugation with Lcn2 and worsens the prognosis of thyroid cancers of anaplastic origin [25].
Gastric cancer
High levels of MMP-2, MMP-9, and Lcn2 have been identified in human gastric cancers. Sampieri et al. identified elevated levels of MMP-9 mRNA levels as well as Lcn2-MMP-9 complexes in gastric cancer cells. This has clinical importance since Lcn2 prevents the degradation of MMP-9 when combined. However, further studies are required to ascertain the role of Lcn2 in gastric cancers [26].
Pancreatic cancer
Lcn2 is being reviewed for its potential as a biomarker in pancreatic cancer, suggesting its role in early diagnosis. Moniaux et al. reported a 27-fold increase of Lcn2 in pancreatic cancer cells [27]. While Lcn2 levels were markedly elevated in pancreatic cancer, they were also higher than normal in pancreatitis. Lcn2 levels may be used to detect the early stages of pancreatic cancer, and it may be possible to use Lcn2 as a biomarker in pancreatitis [27]. Recently, Tong et al. observed the function of Lcn2 in mouse models of pancreatic cancer since it is poorly understood [28]. Lcn2 plays a significant role by partly reducing adhesion and invasion via suppression of FAK activation. It also inhibits angiogenesis by reducing levels of VEGF in pancreatic cancer cells [28].
Colorectal cancer
Neoplastic disorders of colorectal origin also affect the barrier function of the mucosa which disrupts intestinal bacterial homeostasis. Lcn2’s ability to bind to bacterial formyl peptides allows it to scavenge bacterial products. Its mechanisms of action were replicated using mRNA in situ hybridization and immunohistochemistry in both inflammatory and neoplastic diseases and healthy colon by Nielson et al. in 1996 [29]. The study reported high levels of epithelial-derived Lcn2 in the inflammatory regions of the colonic epithelium in benign, pre-malignant, and malignant conditions. Lcn2 was especially abundant in the transitional mucosa and in the superficial ulcerated regions. However, Lcn2 was not present in the adjacent lymph nodes and the normal colon only had traces of the anti-inflammatory protein.
Ovarian epithelial cancer
Ovarian cancer is the fifth leading cause of death in women and is the most common gynecological cancer. If diagnosed early, ovarian cancer has a 90% 5-year survival rate, and over 90% of ovarian malignancies are of epithelial origin [30][30]. While the focus of serum marker use has been on CA-125, Lcn2 was detected in human ovarian surface epithelial (HOSE) cells indicating its potential application as a serological marker [32]. Neutrophil counts are significantly higher in tumors of ovarian origin [33]. It is reasonable to correlate Lcn2 expression upregulation in pre-malignant stages owing to the underlying inflammatory process. Cho et al. noted that while healthy ovarian cells were negative for Lcn2 expression, immunoreactivity was observed in tumor cells with evidence of elevated Lcn2 mRNA expression in the cancer cell lines [21, 32]. Lcn2 expression reflects the extent of epithelial differentiation and is lost once tumor differentiation is poor in later stages [21].
Kidneys
Acute kidney injury
Acute kidney injury (AKI) leads to a sudden decline in kidney function owing to injury and is followed by functional and structural alterations. Lcn2 has recently been discovered as a novel acute-phase biomarker released in acute kidney injury [23, 34]. To determine the risk of developing AKI in kidney transplant recipients, the cut-off for urinary NGAL that was observed at 2 h was 204 ng/mL [35].
Chronic kidney disease
The underlying pathophysiology of chronic kidney disease (CKD) has not been entirely established. Recent studies have explored the predictability of the Lcn2 protein during the progression of CKD. As the number of functional nephrons decreases, there is a compensatory growth of the functional nephrons owing to ensuing molecular and cellular events [36]. However, the compensatory growth may result in the development of pathological renal lesions and progression to end-stage renal failure. Many complications occur including fibrosis of the tubulo-interstitium as a consequence of tubular atrophy, and it compromises the renal erythropoietin capacity leading to anemia [37]. Experimental models of CKD incorporate the evolutionary mechanisms of the disease by mimicking renal deterioration. The pathways discovered by the experimental models led to the therapeutic discovery of widely used renal-angiotensin inhibitors [38]. These experimental models are now being used to ascertain the role of the Lcn2 gene in CKD and other renal pathologies.
Induction of Lcn2 by mRNA
The remnant kidney model (RKM) was used in rats and mice to establish evidence of genetic factors in the progression of CKD [39]. Maximal transcriptional induction was conducted using downregulated mRNAs (38%) in mouse strains to record the profiling of gene expression using the RKM. The study conducted by Viau et al. was able to correlate Lcn2 with lesion progression in CKD in both mice and humans [40]. Using real-time PCR, Lcn2 mRNA and protein were observed to rise by tenfold in 2 months following the reduction of nephrons in mice. The surplus of burden represented by Lcn2 was in the proximal convoluted tubules, and some of the proteins and mRNA were isolated in the renal ascending loop of Henle and collecting ducts. On microscopic examination, Lcn2 was isolated in cytoplasmic granules in the subapical zone indicative of its glomerular infiltrate origin. The proximal epithelial cells dilated owing to the ongoing disease process, and the cystic transformation contained in situ Lcn2 and antibodies against Lcn2, providing evidence for endocytosis of Lcn2 and subsequent local synthesis and secretion in the kidneys. The levels of Lcn2 mRNA identified in the kidneys were in direct proportion to the intensity of ongoing tubular damage [40].
Disease severity and progression
Bolignano et al. established the independent and inverse association of urinary and serum Lcn2 with the progression of CKD thereby identifying its contribution as a risk marker for progression [23]. Lcn2 has been observed to provide real-time detection of kidney damage in mouse models, and the urinary protein biomarker is able to reveal the onset and resolution of kidney injury [41]. It also serves as an excellent diagnostic marker for IgA nephropathy, the most common presentation of glomerulonephritis [42]. Lcn2 is one of the most promising biomarkers for acute kidney injury (AKI), and it may be isolated in both urine and plasma. AKI may occur in the intensive care unit, as well as during kidney damage. Lcn2 is also able to identify sub-clinical AKI in the absence of elevated serum creatinine [43]. Lcn2 upregulation observed late after AKI has been linked with progression to CKD [44].
Gastro-intestinal tract
Gut-origin sepsis
Lcn2 serves as an anti-inflammatory in the intestinal tract and regulates the composition of the gut microbiota. It plays a key role in the pathogenesis of sepsis by providing protection to the gut barrier against injury. It maintains homeostasis of the microbiota and exerts antioxidant stress. It also promotes the deactivation of macrophages and induces immune cell apoptosis to terminate systemic hyper-inflammation. Sepsis has been a key concern in the field of critical care, and the World Health Organization recognized the condition as a global health priority in 2017 [45]. Sepsis was re-defined as a life-threatening condition accompanied by organ dysfunction and caused by a dysfunctional response to infection by the host [46]. The gut comprises the epithelium, immune system, and microbiome—all of which are impacted in critically ill patients and are likely to propagate an ongoing pathology in these patients [47]. Gut-origin sepsis is understood by the analogy of the gut acting as the “motor” component in patients with systemic inflammatory response syndrome (SIRS) and/or multiple organ dysfunction syndromes (MODS) [47, 48].
Gut-specific functions of Lcn2
Major protective mechanisms are present in the gut during gut-origin sepsis including (1) restoration of the microbiota homeostasis by promoting decontamination of selective regions in the gut as well as the use of probiotics and (2) protection of the intestinal barrier by providing antioxidants, enteral nutrition, and immune nutrition [49]. Overgrowth of the pathological gut microbiome is termed “dysbiosis.” Lcn2-negative mice demonstrated higher levels of gut bacterial dysbiosis with the major burden of the dysbiosis carried out by gram-negative bacteria. Conversely, germ-free mice had low levels of serum and fecal Lcn2. The levels were increased when contents from the cecum were injected via oral gavage from wild mice. Singh et al. suggest the importance of Lcn2 as a microbiota inducer, and it may serve as a pre-requisite for intestinal homeostasis [17]. Lcn2 is bacteriostatic against Escherichia coli owing to its affinity for catecholate-type siderophores as observed in mouse models [50]. Lcn2 is released by neutrophils and epithelial cells, and it facilitates bacteriostasis by recruiting neutrophils in a paracrine as well as autocrine manner [51]. Resistance has been observed by bacteria against Lcn2 owing to resistance siderophores [52]. The anti-oxidant effects of Lcn2 have been observed in vitro against H2O2 toxicity [53]. This allows Lcn2 to protect the intestinal barrier against oxidative stress and reduce the impact of intestinal barrier injury during sepsis. Lcn2 acts as an inflammatory modulator to prevent the further release of pro-inflammatory cytokines by enhancing phagocytic bacterial clearance in macrophages [54].
Biomarker potential
Lcn2 is being investigated as a diagnostic and prognostic marker in a wide range of diseases including inflammatory and neoplastic conditions [6]. In children, early detection of acute kidney injury (AKI) is established by measuring urinary NGAL in the first 6 h following admission and a cut-off of 50 mg/dL serves as optimal [55]. The definitions of AKI depend on the elevation of serum creatinine, but serum and urinary NGAL are reported to have higher sensitivity. In healthy children, differences in urinary NGAL were observed to be determined by sex or ethnicity. Females were reported to have consistently higher levels of NGAL than males [56].
Low-grade inflammation due to a wide range of disorders including cancer may be ascertained by measuring fecal Lcn2 using ELISA, as observed in the mouse model [14]. Its use allows for non-invasive, sensitive, and cost-effective means to measure intestinal inflammation as described by Chassaing et al. [14]. In mouse models mimicking the progression of chronic kidney disease (CKD), the intensity of tubular damage was directly proportional to the levels of expressed Lcn2 protein and mRNA. Levels of Lcn2 protein may be measured in the urine using ELISA owing to the kidney being a major source of Lcn2 expression and release [40]. While Lcn2 is not ready for use in clinical practice, it is worthwhile to explore its potential in predicting CKD progression, AKI diagnosis, and risk of cardiovascular disease in CKD [57]. In critically ill patients at high risk of developing cardiovascular complications, measurement of urinary NGAL was able to provide earlier detection of AKI as compared to creatinine. Serum and urinary NGAL are elevated 24 h before creatinine rises to lead to timely detection of impending acute myocardial infarction, heart failure, or stroke [58].
Other functions of Lcn2
Lcn-2 is prominent for its contribution as a prominent biomarker during inflammation, ischemia, infection, and AKI. While its role is protective in infections and IBD, it has both beneficial and determining functions in cancer, neurodegenerative diseases, metabolic syndrome, skin, and other renal disorders. Paradoxical effects of Lcn2 on insulin resistance are observed. Iron dysregulation is involved in the development of insulin resistance and suggests the potential adverse role of Lcn2 in obesity. Almost 1/3rd of patients with obesity and metabolic syndrome suffer from iron dysregulation; the condition is known as dysmetabolic iron overload syndrome (DIOS). Lcn2 may be used as a potential therapeutic target for DIOS [59].
Skin epithelial cells and neutrophils are able to upregulate or release their Lcn2 to prevent potential microbial invasion. Lcn2 has a protective role as it facilitates the process of cutaneous wound healing [60]. However, it has a paradoxical detrimental role as it promotes dysregulated keratinocyte differentiation in several skin disorders, primarily psoriasis. Serum and tissue Lcn2 were observed to be elevated in patients with psoriasis. Anti-Lcn2 antibody treatment was shown to alleviate the disease in a mouse model of psoriasis. Molecular analysis may be conducted to isolate Lcn2 protein expression and levels of mRNA expression encoding for Lcn2 [15]. Lcn2 protein levels are measurable by detecting levels in serum, blood, or feces and by quantifying Lcn2 mRNA transcription or translation. Assays may include the identification of lipocalin-2 mRNA expression, protein expression, or protein activity. Determinants of Lcn2 transcription or translation include levels of mRNA, stability of mRNA, degradation of mRNA, and rates of transcription and translation [15].
Therapeutic potential
Breast
Inhibition of Lcn2 may be able to prevent breast cancer tumorigenesis and invasiveness by acting as an inhibitory monoclonal antibody in certain aggressive breast tumors [24].
Kidney
The role of 4-phenyl butyric acid (PBA) in preventing the toxic effects of proteinuria in CKD progression is closely related to increasing proteinuria. Kidney function may be restored following ischemia-induced tissue injury by employing the macrophage-dependent sphingosine-1-phosphate (S1P)–induced downstream release of Lcn2 [61].
Gut
Experimental models lacking Lcn2 exhibited greater sensitivity to bacterial infections including Escherichia coli and endotoxin-induced sepsis [62, 63]. It may be possible to treat the conditions with exogenous administration of recombinant Lcn2, but the therapeutic roles of Lcn2 in critically ill patients are yet to be established.
Systematic findings of current clinical trials
In total, 70 current clinical trials were included in this synthesis, with a total enrollment of 12,185 participants. The trials were to be completed between December 2022 and December 2030, spanning 8 years in total. The various conditions of the clinical trials comprised acute kidney injury, acute ischemic stroke, other heart diseases, chronic kidney disease, chronic obstructive pulmonary disease, diabetes, liver injury, heat stress, pregnancy-induced diabetes mellitus, obesity, endocrine disorders, and also SARS-CoV-2. Lcn2 was assessed in all outcomes with interventions and diagnostics comprising devices for hypothermia, extracorporeal shockwave therapy, biomarker testing, aminophylline, caffeine citrate, dapagliflozin, nitric oxide, vitamin D3, tadalafil, and heat acclimation. The full summary is enlisted in Tables 2 and 3.
Conclusion
Lcn2 has become increasingly relevant in the last few years given its association with many diseases as a prognostic biomarker. As an acute-phase response, Lcn2 modulates cell physiological responses, acting as the bridge between physiology and pathology. Currently, available data for Lcn2 in organs across the body demonstrates its role in physiological and pathological conditions. Importantly, the levels of Lcn2 having a correlation with the severity of disease demonstrate its use as a prognostic biomarker. However, there are gaps in the understanding of Lcn2’s contribution to the underlying pathophysiology of the disease. Further mice models and translational studies exploring functional roles are required to understand the contribution of Lcn2 beyond its biomarker potential. Exploring Lcn2 as a therapeutic target in inflammatory and malignant conditions will require further studies, and research is currently underway.
References
Hraba-Renevey S, Türler H, Kress M, Salomon C, Weil R. SV40-induced expression of mouse gene 24p3 involves a post-transcriptional mechanism. Oncogene. 1989;4:601–8.
Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem ASBMB. 1993;268:10425–32.
Kjeldsen L, Bainton DF, Sengelov H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. 1994;83:799–807.
Flower DR, North ACT, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta (BBA)-Protein Struct Mol Enzymol. Elsevier; 2000;1482:9–24.
Janas RM, Ochocińska A, Śnitko R, Dudka D, Kierkuś J, Teisseyre M, et al. Neutrophil gelatinase-associated lipocalin in blood in children with inflammatory bowel disease. J Gastroenterol Hepatol Wiley Online Library. 2014;29:1883–9.
Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta (BBA)-Reviews Cancer. Elsevier; 2012;1826:129–69.
Cowland JB, Sørensen OE, Sehested M, Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1β, but not by TNF-α. J Immunol Am Assoc Immnol. 2003;171:6630–9.
Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nat Nat Publ Group. 2004;432:917–21.
Schlehuber S, Skerra A. Lipocalins in drug discovery: from natural ligand-binding proteins to ‘anticalins.’ Drug Discov Today Elsevier. 2005;10:23–33.
Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Sci (80-) Am Assoc Adv Sci. 2001;293:829–34.
Yan Q-W, Yang Q, Mody N, Graham TE, Hsu C-H, Xu Z, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes Am Diabetes Assoc. 2007;56:2533–40.
Hau CS, Kanda N, Tada Y, Shibata S, Uozaki H, Fukusato T, et al. Lipocalin-2 exacerbates psoriasiform skin inflammation by augmenting T-helper 17 response. J Dermatol Wiley Online Library. 2016;43:785–94.
Xiao X, Yeoh BS, Vijay-Kumar M. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu Rev Nutr Annual Reviews. 2017;37:103–30.
Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. USA: Public Library of Science San Francisco; 2012.
Abella V, Scotece M, Conde J, Gómez R, Lois A, Pino J, et al. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers Taylor & Francis. 2015;20:565–71.
Weinberg ED. The Lactobacillus anomaly: total iron abstinence. Perspect Biol Med Johns Hopkins Univ Press. 1997;40:578–83.
Singh V, San Yeoh B, Chassaing B, Zhang B, Saha P, Xiao X, et al. Microbiota-inducible innate immune siderophore binding protein lipocalin 2 is critical for intestinal homeostasis. Cell Mol Gastroenterol Hepatol Elsevier. 2016;2:482–98.
Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest Am Soc Clin Investig. 2007;117:3909–21.
de Villiers WJS, Varilek GW, de Beer FC, Guo J-T, Kindy MS. Increased serum amyloid a levels reflect colitis severity and precede amyloid formation in IL-2 knockout mice. Cytokine Elsevier. 2000;12:1337–47.
Chambers RE, Stross P, Barry RE, Whicher JT. Serum amyloid A protein compared with C-reactive protein, alpha 1-antichymotrypsin and alpha 1-acid glycoprotein as a monitor of inflammatory bowel disease. Eur J Clin Invest Wiley Online Libr. 1987;17:460–7.
Cho H, Kim J-H. Lipocalin 2 expressions correlate significantly with tumor differentiation in epithelial ovarian cancer. J Histochem Cytochem SAGE Publications Sage CA: Los Angeles, CA. 2009;57:513–21.
Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci Nal Acad Sci. 2009;106:3913–8.
Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol Am Soc Nephrol. 2009;4:337–44.
Leng X, Ding T, Lin H, Wang Y, Hu L, Hu J, et al. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res AACR. 2009;69:8579–84.
Iannetti A, Pacifico F, Acquaviva R, Lavorgna A, Crescenzi E, Vascotto C, et al. The neutrophil gelatinase-associated lipocalin (NGAL), a NF-κB-regulated gene, is a survival factor for thyroid neoplastic cells. Proc Natl Acad Sci Natl Acad Sci. 2008;105:14058–63.
Sampieri CL, De La Peña S, Ochoa-Lara M, Zenteno-Cuevas R, León-Córdoba K. Expression of matrix metalloproteinases 2 and 9 in human gastric cancer and superficial gastritis. World J Gastroenterol WJG Baishideng Publ Group Inc. 2010;16:1500.
Moniaux N, Chakraborty S, Yalniz M, Gonzalez J, Shostrom VK, Standop J, et al. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer Nature Publ Group. 2008;98:1540–7.
Tong Z, Kunnumakkara AB, Wang H, Matsuo Y, Diagaradjane P, Harikumar KB, et al. Neutrophil gelatinase–associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res AACR. 2008;68:6100–8.
Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut BMJ Publ Group. 1996;38:414–20.
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin Wiley Online Libr. 2018;68:284–96.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin Wiley Online Libr. 2007;57:43–66.
Cho H, Kang ES, Hong SW, Oh YJ, Choi SM, Kim SW, et al. Genomic and proteomic characterization of YDOV-157, a newly established human epithelial ovarian cancer cell line. Mol Cell Biochem Springer. 2008;319:189–201.
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother Springer. 2009;58:15–23.
Noto A, Cibecchini F, Fanos V, Mussap M. NGAL and metabolomics: the single biomarker to reveal the metabolome alterations in kidney injury. Biomed Res Int. Hindawi; 2013;2013:612032. https://doi.org/10.1155/2013/612032.
Rostami Z, Nikpoor M, Einollahi B. Urinary neutrophil gelatinase associated lipocalin (NGAL) for early diagnosis of acute kidney injury in renal transplant recipients. Nephrourol Mon Kowsar Med Inst. 2013;5:745.
Hostetter TH. Progression of renal disease and renal hypertrophy. Annu Rev Physiol. Annual Reviews 4139 El Camino Way, PO Box 10139, Palo Alto, CA 94303–0139, USA; 1995;57:263–78. https://doi.org/10.1146/annurev.ph.57.030195.001403.
Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim care Clin Off Pract Elsevier. 2008;35:329–44.
Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest Am Soc Clin Investig. 2006;116:288–96.
Ma L-J, Fogo AB. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int Elsevier. 2003;64:350–5.
Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest Am Soc Clin Investig. 2010;120:4065–76.
Paragas N, Qiu A, Zhang Q, Samstein B, Deng S-X, Schmidt-Ott KM, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med Nat Publ Group. 2011;17:216–22.
Ding H, He Y, Li K, Yang J, Li X, Lu R, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin Immunol Elsevier. 2007;123:227–34.
Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med De Gruyter. 2012;50:1505–17.
Ko GJ, Grigoryev DN, Linfert D, Jang HR, Watkins T, Cheadle C, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. MD: Am J Physiol Physiol. American Physiological Society Bethesda; 2010.
Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority—a WHO resolution. N Engl J Med Mass Med Soc. 2017;377:414–7.
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama Am Med Assoc. 2016;315:775–87.
Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin Elsevier. 2016;32:203–12.
Otani S, Coopersmith CM. Gut integrity in critical illness. J intensive care Springer. 2019;7:1–7.
Assimakopoulos SF, Triantos C, Thomopoulos K, Fligou F, Maroulis I, Marangos M, et al. Gut-origin sepsis in the critically ill patient: pathophysiology and treatment. Infect Springer. 2018;46:751–60.
Mori K, Suzuki T, Minamishima S, Igarashi T, Inoue K, Nishimura D, et al. Neutrophil gelatinase-associated lipocalin regulates gut microbiota of mice. J Gastroenterol Hepatol Wiley Online Libr. 2016;31:145–54.
Li H, Feng D, Cai Y, Liu Y, Xu M, Xiang X, et al. Hepatocytes and neutrophils cooperatively suppress bacterial infection by differentially regulating lipocalin-2 and neutrophil extracellular traps. Hepatology Wiley Online Libr. 2018;68:1604–20.
Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio S-P, Paixao TA, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe Elsevier. 2009;5:476–86.
Roudkenar MH, Halabian R, Ghasemipour Z, Roushandeh AM, Rouhbakhsh M, Nekogoftar M, et al. Neutrophil gelatinase-associated lipocalin acts as a protective factor against H2O2 toxicity. Arch Med Res Elsevier. 2008;39:560–6.
Toyonaga T, Matsuura M, Mori K, Honzawa Y, Minami N, Yamada S, et al. Lipocalin 2 prevents intestinal inflammation by enhancing phagocytic bacterial clearance in macrophages. Sci Rep Nat Publ Group. 2016;6:1–13.
Izadi A, Yousefifard M, Nakhjavan-Shahraki B, Baikpour M, Mirzay Razaz J, Hosseini M. Diagnostic value of Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) in detection of pediatric acute kidney injury; a systematic review and meta-analysis. Int J Pediatr. 2016;4:3875–95.
McWilliam SJ, Antoine DJ, Sabbisetti V, Pearce RE, Jorgensen AL, Lin Y, et al. Reference intervals for urinary renal injury biomarkers KIM-1 and NGAL in healthy children. Biomark Med Future Med. 2014;8:1189–97.
Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int Elsevier. 2011;80:806–21.
Helanova K, Spinar J, Parenica J. Diagnostic and prognostic utility of neutrophil gelatinase-associated lipocalin (NGAL) in patients with cardiovascular diseases-review. Kidney Blood Press Res Karger Publishers. 2014;39:623–9.
Wang Y, Lam KSL, Kraegen EW, Sweeney G, Zhang J, Tso AWK, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem Oxford Univ Press. 2007;53:34–41.
Miao Q, Ku AT, Nishino Y, Howard JM, Rao AS, Shaver TM, et al. Tcf3 promotes cell migration and wound repair through regulation of lipocalin 2. Nat Commun Nat Publ Group. 2014;5:1–15.
Sola A, Weigert A, Jung M, Vinuesa E, Brecht K, Weis N, et al. Sphingosine-1-phosphate signalling induces the production of Lcn-2 by macrophages to promote kidney regeneration. J Pathol Wiley Online Libr. 2011;225:597–608.
Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci National Acad Sci. 2006;103:1834–9.
Srinivasan G, Aitken JD, Zhang B, Carvalho FA, Chassaing B, Shashidharamurthy R, et al. Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin-induced sepsis. J Immunol Am Assoc Immnol. 2012;189:1911–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asaf, S., Maqsood, F., Jalil, J. et al. Lipocalin 2—not only a biomarker: a study of current literature and systematic findings of ongoing clinical trials. Immunol Res 71, 287–313 (2023). https://doi.org/10.1007/s12026-022-09352-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09352-2