Abstract
Purpose
To evaluate the effect and mechanism of 1,25(OH)2D3 on pancreatic stellate cells (PSCs) in type 2 diabetes mellitus (T2DM).
Methods
A mouse model of T2DM was successfully established by high-fat diet (HFD) /streptozotocin (STZ) and administered 1,25(OH)2D3 for 3 weeks. Fasting blood glucose (FBG), glycated hemoglobin A1c (GHbA1c), insulin (INS) and glucose tolerance were measured. Histopathology changes and fibrosis of pancreas were examined by hematoxylin and eosin staining and Masson staining. Mouse PSCs were extracted, co-cultured with mouse insulinoma β cells (MIN6 cells) and treated with 1,25(OH)2D3. ELISA detection of inflammatory factor expression. Tissue reactive oxygen species (ROS) levels were also measured. Immunofluorescence or Western blotting were used to measure fibrosis and inflammation-related protein expression.
Results
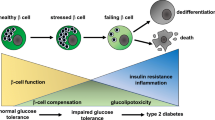
PSCs activation and islets fibrosis in T2DM mice. Elevated blood glucose was accompanied by significant increases in serum inflammatory cytokines and tissue ROS levels. 1,25(OH)2D3 attenuated islet fibrosis by reducing hyperglycemia, ROS levels, and inflammatory factors expression. Additionally, the co-culture system confirmed that 1,25(OH)2D3 inhibited PSCs activation, reduced the secretion of pro-inflammatory cytokines, down-regulated the expression of fibrosis and inflammation-related proteins, and promoted insulin secretion.
Conclusion
Our findings identify that PSCs activation contributes to islet fibrosis and β-cell dysfunction. 1,25(OH)2D3 exerts beneficial effects on T2DM potentially by inhibiting PSCs activation and inflammatory response, highlighting promising control strategies of T2DM by vitamin D.
Graphical Abstract

Fig. 7 Molecular mechanism of 1,25(OH)2D3 in β cell damage following PSCs activation in T2DM mice.
Highlights
-
PSCs activation leads to islet fibrosis in T2DM mice.
-
TGF- β, IL-1 β, TNF-α, ROS induce PSCs activation, thereby inhibiting MIN6 cell proliferation and function.
-
1,25(OH)2D3 inhibits PSCs activation by downregulating the expression of factors such as TGF-β, protecting β cell function.






Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
T.I.A. Sorensen, S. Metz, T.O. Kilpelainen, Do gene-environment interactions have implications for the precision prevention of type 2 diabetes? Diabetologia 65, 1804–1813 (2022). https://doi.org/10.1007/s00125-021-05639-5
N.J. Pillon, R.J.F. Loos, S.M. Marshall, J.R. Zierath, Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell 184, 1530–1544 (2021). https://doi.org/10.1016/j.cell.2021.02.012
M.A. Nauck, J. Wefers, J.J. Meier, Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol. 9, 525–544 (2021). https://doi.org/10.1016/S2213-8587(21)00113-3
Y. Wu, C. Zhang, K. Jiang, J. Werner, A.V. Bazhin, J.G. D’Haese, The Role of Stellate Cells in Pancreatic Ductal Adenocarcinoma: Targeting Perspectives. Front. Oncol. 10, 621937 (2020). https://doi.org/10.3389/fonc.2020.621937
Y. Wu, C. Zhang, M. Guo, W. Hu, Y. Qiu, M. Li, D. Xu, P. Wu, J. Sun, R. Shi, Z. Zhang, K. Jiang, Targeting pancreatic stellate cells in chronic pancreatitis: Focus on therapeutic drugs and natural compounds. Front. Pharmacol. 13, 1042651 (2022). https://doi.org/10.3389/fphar.2022.1042651
A. Allam, A.R. Thomsen, M. Gothwal, D. Saha, J. Maurer, T.B. Brunner, Pancreatic stellate cells in pancreatic cancer: In focus. Pancreatology 17, 514–522 (2017). https://doi.org/10.1016/j.pan.2017.05.390
R.R. Bynigeri, A. Jakkampudi, R. Jangala, C. Subramanyam, M. Sasikala, G.V. Rao, D.N. Reddy, R. Talukdar, Pancreatic stellate cell: Pandora’s box for pancreatic disease biology. World J. Gastroenterol. 23, 382–405 (2017). https://doi.org/10.3748/wjg.v23.i3.382
E. Lee, G.R. Ryu, S.H. Ko, Y.B. Ahn, K.H. Song, A role of pancreatic stellate cells in islet fibrosis and beta-cell dysfunction in type 2 diabetes mellitus. Biochem. Biophys. Res. Commun. 485, 328–334 (2017). https://doi.org/10.1016/j.bbrc.2017.02.082
P. Hrabak, M. Kalousova, T. Krechler, T. Zima, Pancreatic stellate cells - rising stars in pancreatic pathologies. Physiol. Res. 70, S597–S616 (2021). https://doi.org/10.33549/physiolres.934783
Y. Yang, J.W. Kim, H.S. Park, E.Y. Lee, K.H. Yoon, Pancreatic stellate cells in the islets as a novel target to preserve the pancreatic beta-cell mass and function. J. Diabetes Investig. 11, 268–280 (2020). https://doi.org/10.1111/jdi.13202
A. Hanel, C. Carlberg, Vitamin D and evolution: Pharmacologic implications. Biochem. Pharmacol. 173, 113595 (2020). https://doi.org/10.1016/j.bcp.2019.07.024
M.E. de Albuquerque Borborema, D.C. Oliveira, J. de Azevedo Silva, Down regulation of VDR gene expression in metabolic syndrome and atherosclerosis’ patients: Cause or consequence? Gene 771, 145341 (2021). https://doi.org/10.1016/j.gene.2020.145341
M. Zheng, R. Gao, Vitamin D: A Potential Star for Treating Chronic Pancreatitis. Front. Pharmacol. 13, 902639 (2022). https://doi.org/10.3389/fphar.2022.902639
A. Sacerdote, P. Dave, V. Lokshin, G. Bahtiyar, Type 2 Diabetes Mellitus, Insulin Resistance, and Vitamin D. Curr. Diab. Rep. 19, 101 (2019). https://doi.org/10.1007/s11892-019-1201-y
H. Lim, H. Lee, Y. Lim, Effect of vitamin D(3) supplementation on hepatic lipid dysregulation associated with autophagy regulatory AMPK/Akt-mTOR signaling in type 2 diabetic mice. Exp. Biol. Med. (Maywood) 246, 1139–1147 (2021). https://doi.org/10.1177/1535370220987524
H.L. Zhao, F.M. Lai, P.C. Tong, D.R. Zhong, D. Yang, B. Tomlinson, J.C. Chan, Prevalence and clinicopathological characteristics of islet amyloid in Chinese patients with type 2 diabetes. Diabetes 52, 2759–2766 (2003). https://doi.org/10.2337/diabetes.52.11.2759
H. Lee, Y. Lim, Tocotrienol-rich fraction supplementation reduces hyperglycemia-induced skeletal muscle damage through regulation of insulin signaling and oxidative stress in type 2 diabetic mice. J. Nutr. Biochem. 57, 77–85 (2018). https://doi.org/10.1016/j.jnutbio.2018.03.016
W. Luo, Y. Jiang, Z. Yi, Y. Wu, P. Gong, Y. Xiong, 1ɑ,25-Dihydroxyvitamin D(3) promotes osteogenesis by down-regulating FGF23 in diabetic mice. J. Cell Mol. Med. 25, 4148–4156 (2021). https://doi.org/10.1111/jcmm.16384
S.A. Blaine, K.C. Ray, K.M. Branch, P.S. Robinson, R.H. Whitehead, A.L. Means, Epidermal growth factor receptor regulates pancreatic fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G434–441 (2009). https://doi.org/10.1152/ajpgi.00152.2009
I. Tuleta, N.G. Frangogiannis, Diabetic fibrosis. Biochim. Biophys. Acta. Mol. Basis Dis. 1867, 166044 (2021). https://doi.org/10.1016/j.bbadis.2020.166044
A. Rojas, C. Añazco, I. González, P. Araya, n: a missing piece in the puzzle of the association between diabetes and cancer. Carcinogenesis 39, 515–521 (2018). https://doi.org/10.1093/carcin/bgy012
Y. Jin, K. Ratnam, P.Y. Chuang, Y. Fan, Y. Zhong, Y. Dai, A.R. Mazloom, E.Y. Chen, V. D’Agati, H. Xiong, M.J. Ross, N. Chen, A. Ma’ayan, J.C. He, A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat. Med. 18, 580–588 (2012). https://doi.org/10.1038/nm.2685
M.S. Shah, M. Brownlee, Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 118, 1808–1829 (2016). https://doi.org/10.1161/CIRCRESAHA.116.306923
Y. An, H. Zhang, C. Wang, F. Jiao, H. Xu, X. Wang, W. Luan, F. Ma, L. Ni, X. Tang, M. Liu, W. Guo, L. Yu, Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. Faseb J. 33, 12515–12527 (2019). https://doi.org/10.1096/fj.201802805RR
L. Mateus Gonçalves, E. Pereira, J.P. Werneck de Castro, E. Bernal-Mizrachi, J. Almaça, Islet pericytes convert into profibrotic myofibroblasts in a mouse model of islet vascular fibrosis. Diabetologia 63, 1564–1575 (2020). https://doi.org/10.1007/s00125-020-05168-7
L. Tian, Z.P. Lu, B.B. Cai, L.T. Zhao, D. Qian, Q.C. Xu, P.F. Wu, Y. Zhu, J.J. Zhang, Q. Du, Y. Miao, K.R. Jiang, Activation of pancreatic stellate cells involves an EMT-like process. Int. J. Oncol. 48, 783–792 (2016). https://doi.org/10.3892/ijo.2015.3282
H.J. Maier, U. Schmidt-Strassburger, M.A. Huber, E.M. Wiedemann, H. Beug, T. Wirth, NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 295, 214–228 (2010). https://doi.org/10.1016/j.canlet.2010.03.003
B. Sutariya, D. Jhonsa, M.N. Saraf, TGF-β: the connecting link between nephropathy and fibrosis. Immunopharmacol. Immunotoxicol. 38, 39–49 (2016). https://doi.org/10.3109/08923973.2015.1127382
J. Zhang, J. Bai, Q. Zhou, Y. Hu, Q. Wang, L. Yang, H. Chen, H. An, C. Zhou, Y. Wang, X. Chen, M. Li, Glutathione prevents high glucose-induced pancreatic fibrosis by suppressing pancreatic stellate cell activation via the ROS/TGFβ/SMAD pathway. Cell Death Dis. 13, 440 (2022). https://doi.org/10.1038/s41419-022-04894-7
X.F. Xu, F. Liu, J.Q. Xin, J.W. Fan, N. Wu, L.J. Zhu, L.F. Duan, Y.Y. Li, H. Zhang, Respective roles of the mitogen-activated protein kinase (MAPK) family members in pancreatic stellate cell activation induced by transforming growth factor-β1 (TGF-β1). Biochem. Biophys. Res. Commun. 501, 365–373 (2018). https://doi.org/10.1016/j.bbrc.2018.04.176
S Ding, S. Xu, Y. Ma, G. Liu, H. Jang, J. Fang, Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules 9 (2019). https://doi.org/10.3390/biom9120850.
D. He, Y. Wang, R. Liu, A. He, S. Li, X. Fu, Z. Zhou, 1,25(OH)(2)D(3) Activates Autophagy to Protect against Oxidative Damage of INS-1 Pancreatic Beta Cells. Biol. Pharm. Bull. 42, 561–567 (2019). https://doi.org/10.1248/bpb.b18-00395
S. Jiang, H. Zhang, X. Li, B. Yi, L. Huang, Z. Hu, A. Li, J. Du, Y. Li, W. Zhang, Vitamin D/VDR attenuate cisplatin-induced AKI by down-regulating NLRP3/Caspase-1/GSDMD pyroptosis pathway. J. Steroid. Biochem. Mol. Biol. 206, 105789 (2021). https://doi.org/10.1016/j.jsbmb.2020.105789
Acknowledgements
Funding from the National Natural Science Foundation of China (grant numbers 31970505) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.Z.; Data curation, X.W.; Formal analysis, X.W.; A.W.; Funding acquisition, Z.Z.; Investigation, L.Z., X.W. and Y.Z.; Methodology, X.W. and Z.Z.; Project administration, Z.Z.; Resources, X.W., Z.Z., and A.W.; Software, X.W.; Supervision, Z.Z.; Validation, L.Z., X.W., A.W., and Y.Z.; Visualization, L.Z., X.W. and A.W.; Writing – original draft, L.Z., X.W., Y.H., M.X., and Y.Z.; Writing – review & editing, L.Z., Y.H., M.X. A.W., and Z.Z. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All of the experimental procedures involving animals were conducted in accordance with the Institutional Animal Care guidelines of Soochow University, China and approved by the Ethics Committee of Soochow University, Jiangsu Province, China.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Zhang, L., Wei, X. et al. 1,25(OH)2D3 inhibits pancreatic stellate cells activation and promotes insulin secretion in T2DM. Endocrine (2024). https://doi.org/10.1007/s12020-024-03833-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03833-0