Abstract
Purpose of Review
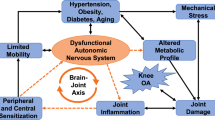
The autonomic nervous system is an important regulator of stress responses and exhibits functional changes in chronic pain states. This review discusses potential overlap among autonomic dysregulation, osteoarthritis (OA) progression, and chronic pain. From this foundation, we then discuss preclinical to clinical research opportunities to close gaps in our knowledge of autonomic dysregulation and OA. Finally, we consider the potential to generate new therapies for OA pain via modulation of the autonomic nervous system.
Recent Findings
Recent reviews provide a framework for the autonomic nervous system in OA progression; however, research is still limited on the topic. In other chronic pain states, functional overlaps between the central autonomic network and pain processing centers in the brain suggest relationships between concomitant dysregulation of the two systems. Non-pharmacological therapeutics, such as vagus nerve stimulation, mindfulness-based meditation, and exercise, have shown promise in alleviating painful symptoms of joint diseases, and these interventions may be partially mediated through the autonomic nervous system.
Summary
The autonomic nervous system appears to be dysregulated in OA progression, and further research on rebalancing autonomic function may lead to novel therapeutic strategies for treating OA pain.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO Department of Health Statistics and Information Systems (2013) World Health Organization methods and data sources for global burden of disease estimates 2000–2011. Geneva
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707.
Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, Campbell CM, Haythornthwaite JA, Edwards RR, Smith MT. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65:363–72.
Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–89.
Pre-Competitive Consortium for Osteoarthritis of the Osteoarthritis Research International (2016) Osteoarthritis: a serious disease, submitted to the U.S. Food and Drug Administration.
Lanza FL, Chan FKL, Quigley EMM, et al. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–38.
Liu G, Yan YP, Zheng XX, Xu YL, Lu J, Hui RT, Huang XH. Meta-analysis of nonsteroidal anti-inflammatory drug use and risk of atrial fibrillation. Am J Cardiol. 2014;114:1523–9.
Hsu CC, Wang H, Hsu YH, Chuang SY, Huang YW, Chang YK, Liu JS, Hsiung CA, Tsai HJ. Use of nonsteroidal anti-inflammatory drugs and risk of chronic kidney disease in subjects with hypertension: nationwide longitudinal cohort study. Hypertension. 2015;66:524–33.
Andriacchi TP, Griffin TM, Loeser RF, Chu CR, Roos EM, Hawker GA, Erhart-Hledik JC, Fischer AG (2020) Bridging disciplines as a pathway to finding new solutions for osteoarthritis a collaborative program presented at the 2019 Orthopaedic Research Society and the Osteoarthritis Research Society International. Osteoarthritis and Cartilage Open 2:100026
Courties A, Sellam J, Berenbaum F. Role of the autonomic nervous system in osteoarthritis. Best Pract Res Clin Rheumatol. 2017;31:661–75. Provides framework for the contributions of the autonomic nervous system to osteoarthritis pathophysiology
Berenbaum F, Meng QJ. The brain-joint axis in osteoarthritis: nerves, circadian clocks and beyond. Nat Rev Rheumatol. 2016;12:508–16.
Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–54.
Boscan P, Kasparov S, Paton JFR. Somatic nociception activates NK1 receptors in the nucleus tractus solitarii to attenuate the baroreceptor cardiac reflex. Eur J Neurosci. 2002;16:907–20.
Pickering AE, Boscan P, Paton JFR. Nociception attenuates parasympathetic but not sympathetic baroreflex via NK1 receptors in the rat nucleus tractus solitarii. J Physiol. 2003;551:589–99.
Rotenberg S, McGrath JJ. Inter-relation between autonomic and HPA axis activity in children and adolescents. Biol Psychol. 2016;117:16–25.
Villafañe JH, et al. Exploring the relationship between chronic pain and cortisol levels in subjects with osteoarthritis: results from a systematic review of the literature. Osteoarthritis Cartilage. 2020;28(5):572–80.
Yang X, Qi Y, Avercenc-Leger L, Vincourt JB, Hupont S, Huselstein C, Wang H, Chen L, Magdalou J (2017) Effect of nicotine on the proliferation and chondrogenic differentiation of the human Wharton’s jelly mesenchymal stem cells. In: Bio-medical materials and engineering. Biomed Mater Eng, pp S217–S228
Kawakita A, Sato K, Makino H, Ikegami H, Takayama S, Toyama Y, Umezawa A. Nicotine acts on growth plate chondrocytes to delay skeletal growth through the α7 neuronal nicotinic acetylcholine receptor. PLoS ONE. 2008;3:e3945.
Liu Y, Wu D, Song F, Zhu C, Hui Y, Zhu Q, Wu J, Fan W, Hu J. Activation of α7 nicotinic acetylcholine receptors prevents monosodium iodoacetate-induced osteoarthritis in rats. Cell Physiol Biochem. 2015;35:627–38.
Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, Kawashima K. Expression and function of the cholinergic system in immune cells. Front Immunol. 2017. https://doi.org/10.3389/fimmu.2017.01085.
Chisari E, Wouthuyzen-Bakker M, Friedrich AW, Parvizi J. The relation between the gut microbiome and osteoarthritis: a systematic review of literature. PLoS ONE. 2021;16(12):e0261353.
Minerbi A, et al. Altered microbiome composition in individuals with fibromyalgia. Pain. 2019;160(11):2589–602.
Martins DF, et al. The role of the vagus nerve in fibromyalgia syndrome. Neurosci Biobehav Rev. 2021;131:1136–49.
Gupta VK, et al. Gut microbial determinants of clinically important improvement in patients with rheumatoid arthritis. Genome Med. 2021;13(1):1–20.
Ingegnoli F, Buoli M, Antonucci F, Coletto LA, Esposito CM, Caporali R. The link between autonomic nervous system and rheumatoid arthritis: from bench to bedside. Front Med. 2020;7:859.
Dekker Nitert M, Mousa A, Barrett HL, Naderpoor N, de Courten B. Altered gut microbiota composition is associated with back pain in overweight and obese individuals. Front Endocrinol (Lausanne). 2020;11:605.
El-Badawy MA, El Mikkawy DME. Sympathetic dysfunction in patients with chronic low back pain and failed back surgery syndrome. Clin J Pain. 2016;32(3):226–31.
Bassi GS, Dias DPM, Franchin M, et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav Immun. 2017;64:330–43.
Konttinen YT, Sillat T, Barreto G, Ainola M, Nordström DCE. Osteoarthritis as an autoinflammatory disease caused by chondrocyte-mediated inflammatory responses. Arthritis Rheum. 2012;64:613–6.
Lorenz J, Schäfer N, Bauer R, Jenei-Lanzl Z, Springorum RH, Grässel S. Norepinephrine modulates osteoarthritic chondrocyte metabolism and inflammatory responses. Osteoarthritis Cartilage. 2016;24:325–34.
Courties A, Do A, Leite S, et al. The role of the non-neuronal cholinergic system in inflammation and degradation processes in osteoarthritis. Arthritis Rheumatol. 2020;72:2072–82.
Bock K, Plaass C, Coger V, Peck C-T, Reimers K, Stukenborg-Colsman C, Claassen L. What is the effect of nicotinic acetylcholine receptor stimulation on osteoarthritis in a rodent animal model? SAGE Open Med. 2016;4:205031211663752.
Courties A, Do A, Leite S, et al. Activating the cholinergic system a novel opportunity for treating osteoarthritis. Osteoarthritis Cartilage. 2019;27:S38.
Teng P, Liu Y, Dai Y, Zhang H, Liu W-T, Hu J. Nicotine attenuates osteoarthritis pain and matrix metalloproteinase-9 expression via the α7 nicotinic acetylcholine receptor. J Immunol. 2019;203:485–92.
Louati K, Berenbaum F. Fatigue in chronic inflammation—a link to pain pathways. Arthritis Res Ther. 2015. https://doi.org/10.1186/s13075-015-0784-1.
Farzi A, Fröhlich EE, Holzer P. Gut microbiota and the neuroendocrine system. Neurotherapeutics. 2018;15:5–22.
Franciosi S, Perry FKG, Roston TM, Armstrong KR, Claydon VE, Sanatani S. The role of the autonomic nervous system in arrhythmias and sudden cardiac death. Auton Neurosci: Basic Clin. 2017;205:1–11.
Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248:188–204.
Provan SA, Olstad DS, Solberg EE, Smedslund G, Dagfinrud H. Evidence of reduced parasympathetic autonomic regulation in inflammatory joint disease: a meta-analyses study. Semin Arthritis Rheum. 2018;48:134–40.
Adlan AM, Veldhuijzen van Zanten JJCS, Lip GYH, Paton JFR, Kitas GD, Fisher JP. Cardiovascular autonomic regulation, inflammation and pain in rheumatoid arthritis. Auton Neurosci: Basic Clin. 2017;208:137–45.
van Maanen MA, Stoof SP, LaRosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis. 2010;69:1717–23.
Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, Wilder RL. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci USA. 1989;86:2374–8.
Yeater TD, Zubcevic J, Allen KD Measures of cardiovascular function suggest autonomic nervous system dysregulation after surgical induction of joint injury in the male Lewis rat. Osteoarthritis and Cartilage (In Press):
Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology (United Kingdom). 2018;57:iv43–50.
McDougall J. Arthritis and pain: neurogenic origin of joint pain. Arthritis Res Ther. 2006. https://doi.org/10.1186/ar2069.
Malfait AM, Miller RJ. Emerging targets for the management of osteoarthritis pain. Curr Osteoporos Rep. 2016;14:260–8.
Eitner A, Hofmann GO, Schaible H-G, Kress M, Cunha TM. Mechanisms of osteoarthritic pain. Stud Hum Exp Models. 2017;10:1–22.
Schaible HG. Osteoarthritis pain. Recent advances and controversies. Curr Opin Support Palliat Care. 2018;12:148–53.
McDougall JJ, Bray RC, Sharkey KA. Morphological and immunohistochemical examination of nerves in normal and injured collateral ligaments of rat, rabbit, and human knee joints. Anat Rec. 1997;248:29–39.
Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–66.
Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–9.
O’Connor BL. The mechanoreceptor innervation of the posterior attachments of the lateral meniscus of the dog knee joint. J Anat. 1984;138:15–26.
Freeman MAR, Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat. 1967;101:505.
Marinozzi G, Ferrante F, Goudio E, Ricci A, Amenta F. Intrinsic innervation of the rat knee joint articular capsule and ligaments. Acta Anat (Basel). 1991;141:8–14.
Schuelert N, McDougall JJ. Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett. 2009;465:184–8.
O’Brien MS, McDougall JJ. Neurophysiological assessment of joint nociceptors in the rat medial meniscus transection model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2020;28:1255–64.
Cortelli P, Giannini G, Favoni V, Cevoli S, Pierangeli G. Nociception and autonomic nervous system. Neurol Sci. 2013;34:S41–6.
Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011. https://doi.org/10.1016/j.pain.2010.09.030.
Thakur M, Rahman W, Hobbs C, Dickenson AH, Bennett DLH. Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0033730.
Terry EL, Booker SQ, Cardoso JS, et al. Neuropathic-like pain symptoms in a community-dwelling sample with or at risk for knee osteoarthritis. Pain Med (United States). 2020;21:125–37.
Leone M, Proietti Cecchini A, Mea E, Tullo V, Curone M, Bussone G. Neuroimaging and pain: a window on the autonomic nervous system. Neurol Sci. 2006;27:s134–7.
Heinricher MM, Fields HL (2013) Central nervous system mechanisms of pain modulation. Wall and Melzack’s textbook of pain 6:129–142
Hohenschurz-Schmidt DJ, Calcagnini G, Dipasquale O, et al. Linking pain sensation to the autonomic nervous system: the role of the anterior cingulate and periaqueductal gray resting-state networks. Front Neurosci. 2020;14:147. Evidence for centrally mediated functional connections between pain and autonomics
Stroman PW, Ioachim G, Powers JM, Staud R, Pukall C. Pain processing in the human brainstem and spinal cord before, during, and after the application of noxious heat stimuli. Pain. 2018;159:2012–20. Evidence for centrally mediated functional connections between pain and autonomics
Yeater TD, Clark DJ, Hoyos L, Valdes-Hernandez PA, Peraza JA, Allen KD, Cruz-Almeida Y. Chronic pain is associated with reduced sympathetic nervous system reactivity during simple and complex walking tasks: potential cerebral mechanisms. Chronic Stress. 2021. https://doi.org/10.1177/24705470211030273.
Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624.
Barve RA, Minnerly JC, Weiss DJ, Meyer DM, Aguiar DJ, Sullivan PM, Weinrich SL, Head RD. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: relevance to human disease. Osteoarthritis Cartilage. 2007;15:1190–8.
Aksentijevich S, Whitfield HJ, Scott Young W, Wilder RL, Chrousos GP, Gold PW, Sternberg EM. Arthritis-susceptible Lewis rats fail to emerge from the stress hyporesponsive period. Dev Brain Res. 1992;65:115–8.
Chaouloff F, Kulikov A, Sarrieau A, Castanon N, Mormède P. Male Fischer 344 and Lewis rats display differences in locomotor reactivity, but not in anxiety-related behaviours: relationship with the hippocampal serotonergic system. Brain Res. 1995;693:169–78.
Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010. https://doi.org/10.1016/j.joca.2010.05.030.
E. Macfarlane, M. J. Seibel, and H. Zhou, “Arthritis and the role of endogenous glucocorticoids,” Bone Res. 8 1 2020.
Kenwood MM, Kalin NH, Barbas H. The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology. 2022;47:260–75.
Young HM, Cane KN, Anderson CR. Development of the autonomic nervous system: a comparative view. Auton Neurosci: Basic Clin. 2011;165:10–27.
Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. 2016;113:8284–9.
Levine YA, Koopman FA, Faltys M, Caravaca A, Bendele A, Zitnik R, Vervoordeldonk MJ, Tak PP. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0104530.
Marsal S, Corominas H, Lopez Lasanta M, et al. SAT0133 Pilot clinical study of a non-invasive auricular vagus nerve stimulation device in patients with rheumatoid arthritis. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-eular.3315.
Courties A, Deprouw C, Maheu E, Gibert E, Gottenberg J-E, Champey J, Rousseau A, Berenbaum F, Sellam J. Transcutaneous auricular stimulation of the vagus nerve for erosive hand osteoarthritis an open label pilot study. Osteoarthr Cartil. 2020. https://doi.org/10.1016/j.joca.2020.02.562. First study on vagal nerve stimulation in osteoarthritis
Fu Q, Levine BD (2013) Exercise and the autonomic nervous system. In: Handbook of Clinical Neurology. Elsevier 147–160
Thorstensson CA, Henriksson M, von Porat A, Sjödahl C, Roos EM. The effect of eight weeks of exercise on knee adduction moment in early knee osteoarthritis—a pilot study. Osteoarthritis Cartilage. 2007;15:1163–70.
Hunt MA, Birmingham TB, Skarakis-Doyle E, Vandervoort AA. Towards a biopsychosocial framework of osteoarthritis of the knee. Disabil Rehabil. 2008;30:54–61.
van Oosterwijck J, Marusic U, de Wandele I, Paul L, Meeus M, Moorkens G, Lambrecht L, Danneels L, Nijs J. The role of autonomic function in exercise induced endogenous analgesia: a case-control study in myalgic encephalomyelitis⁄chronic fatigue syndrome and healthy people. Pain Physician. 2017;20:E389–99.
Goyal M, Singh S, Sibinga EMS, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357–68.
Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep. 2012;16:147–52.
Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16:213–25.
Lee A, Harvey W, Price L, Morgan L, Morgan N, Wang C. Mindfulness is associated with psychological health and moderates pain in knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:824–31.
Ahn H, Zhong C, Miao H, Chaoul A, Park L, Yen I, Vila M, Sorkpor S, Abdi S. Efficacy of combining home-based transcranial direct current stimulation with mindfulness-based meditation for pain in older adults with knee osteoarthritis: a randomized controlled pilot study. J Clin Neurosci. 2019;70:140–5.
Fillingim R, Woods A, Ahn H, et al. Pain relief for osteoarthritis through combined treatment (PROACT): protocol for a randomized controlled trial of mindfulness meditation combined with transcranial direct current stimulation in non-Hispanic black and white adults with knee osteoarthritis. Contemp Clin Trials. 2020. https://doi.org/10.1016/J.CCT.2020.106159.
Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014;94:1816–25.
Funding
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR071431, R01AR071431S01, R01AR071431S02, and F31 AR077996. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteoarthritis
Rights and permissions
About this article
Cite this article
Yeater, T.D., Cruz, C.J., Cruz-Almeida, Y. et al. Autonomic Nervous System Dysregulation and Osteoarthritis Pain: Mechanisms, Measurement, and Future Outlook. Curr Rheumatol Rep 24, 175–183 (2022). https://doi.org/10.1007/s11926-022-01071-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-022-01071-9