Abstract
Purpose of Review
The purpose of this review was to synthesize the evidence on non-traditional biomarkers from proteomic and metabolomic studies that may distinguish heart failure (HF) with preserved ejection fraction (HFpEF) from heart failure with reduced ejection fraction (HFrEF) and non-HF.
Recent Findings
Understanding the pathophysiology of HFpEF continues to be challenging. A number of inflammatory and metabolic biomarkers that have recently been suggested to be involved include C-reactive protein (CRP), interleukin-6 (IL-6), trimethylamine-N-oxide (TMAO), syndecan-1 (SDC-1), nitric oxide (NO), and tumor necrosis factor receptor-1 (TNFR-1).
Summary
A systematic search was conducted using Medline, EMBASE, and Web of Science with search terms such as “HFpEF,” “metabolomics,” and “proteomics,” and a meta-analysis was conducted. The results demonstrate significantly higher levels of TMAO, CRP, SDC-1, and IL-6 in HFpEF compared to controls without HF and significantly higher levels of TMAO and CRP in HFrEF compared to controls. The results further suggest that HFpEF might be distinguishable from HFrEF based on higher levels of IL-6 and lower levels of SDC-1 and NO. These data may reflect pathophysiological differences between HFpEF and HFrEF.







Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. https://doi.org/10.1161/HHF.0b013e318291329a.
Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. https://doi.org/10.1161/CIRCULATIONAHA.111.080770.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of Amer. Circ (New York, NY). 2017;136:e137–61. https://doi.org/10.1161/cir.0000000000000509.
Shah S. Precision medicine for heart failure with preserved ejection fraction: an overview. J Cardiovasc Transl Res. 2017;10:233–44. https://doi.org/10.1007/s12265-017-9756-y.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61. https://doi.org/10.1056/NEJMoa2107038.
Rabkin SW, Tang JKK. The utility of growth differentiation factor-15, galectin-3, and sST2 as biomarkers for the diagnosis of heart failure with preserved ejection fraction and compared to heart failure with reduced ejection fraction: a systematic review. Heart Fail Rev. 2021;26:799–812. https://doi.org/10.1007/s10741-020-09913-3.
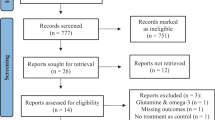
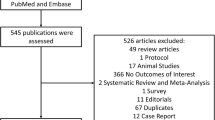
Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Schuett K, Kleber ME, Scharnagl H, Lorkowski S, Marz W, Niessner A, et al. Trimethylamine-N-oxide and heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2017;70:3202–4. https://doi.org/10.1016/j.jacc.2017.10.064.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. 2021 Cochrane handbook for systematic reviews of interventions version 6.2.
Bai B, Cheng M, Jiang L, Xu J, Chen H, Xu Y. High neutrophil to lymphocyte ratio and its gene signatures correlate with diastolic dysfunction in heart failure with preserved ejection fraction. Front Cardiovasc Med. 2021;8:614757. https://doi.org/10.3389/fcvm.2021.614757.
Berge K, Lyngbakken MN, Myhre PL, Brynildsen J, Røysland R, Strand H, et al. High-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide in acute heart failure: data from the ACE 2 study. Clin Biochem. 2021;88:30–6. https://doi.org/10.1016/j.clinbiochem.2020.11.009.
van Wezenbeek J, Canada JM, Ravindra K, Carbone S, Trankle CR, Kadariya D, et al. C-Reactive protein and N-terminal pro-brain natriuretic peptide levels correlate with impaired cardiorespiratory fitness in patients with heart failure across a wide range of ejection fraction . Front Cardiovasc Med 2018;5.
Tromp J, Khan MAF, Mentz RJ, O’Connor CM, Metra M, Dittrich HC, et al. Biomarker profiles of acute heart failure patients with a mid-range ejection fraction. JACC Heart Fail. 2017;5:507–17. https://doi.org/10.1016/j.jchf.2017.04.007.
Brouwers FP, De Boer RA, Van Der Harst P, Voors AA, Gansevoort RT, Bakker SJ, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. https://doi.org/10.1093/eurheartj/eht066.
Kanagala P, Arnold JR, Singh A, Chan DCS, Cheng ASH, Khan JN, et al. Characterizing heart failure with preserved and reduced ejection fraction: an imaging and plasma biomarker approach. PLoS ONE. 2020;15:e0232280. https://doi.org/10.1371/journal.pone.0232280.
Moran A, Katz R, Smith NL, Fried LF, Sarnak MJ, Seliger SL, et al. Cystatin C concentration as a predictor of systolic and diastolic heart failure. J Card Fail. 2008;14:19–26. https://doi.org/10.1016/j.cardfail.2007.09.002.
Sinning C, Kempf T, Schwarzl M, Lanfermann S, Ojeda F, Schnabel RB, et al. Biomarkers for characterization of heart failure - distinction of heart failure with preserved and reduced ejection fraction. Int J Cardiol. 2017;227:272–7. https://doi.org/10.1016/j.ijcard.2016.11.110.
Grossekettler L, Schmack B, Brockmann C, Wanninger R, Kreusser MM, Frankenstein L, et al. Benefits of peritoneal ultrafiltration in HFpEF and HFrEF patients. BMC Nephrol. 2020;21:179. https://doi.org/10.1186/s12882-020-01777-x.
Kang J, Park JJ, Cho Y-J, Oh I-Y, Park H-A, Lee SE, et al. Predictors and prognostic value of worsening renal function during admission in HFpEF versus HFrEF: data from the KorAHF (Korean Acute Heart Failure) registry. J Am Heart Assoc 2018;7. https://doi.org/10.1161/JAHA.117.007910.
Mitic VT, Stojanovic DR, Deljanin Ilic MZ, Stojanovic MM, Petrovic DB, Ignjatovic AM, et al. Cardiac remodeling biomarkers as potential circulating markers of left ventricular hypertrophy in heart failure with preserved ejection fraction. Tohoku J Exp Med. 2020;250:233–42. https://doi.org/10.1620/tjem.250.233.
Tromp J, Khan MAF, Klip IjT, Meyer S, de Boer RA, Jaarsma T, et al. Biomarker profiles in heart failure patients with preserved and reduced ejection fraction. J Am Heart Assoc 2017;6. https://doi.org/10.1161/JAHA.116.003989.
Janeiro MH, Ramirez MJ, Milagro FI, Martinez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10:1398. https://doi.org/10.3390/nu10101398. This review article discussed dietary sources and metabolic pathways of trimethylamine-N-oxide (TMAO), the possible involvement of TMAO in the etiology of cardiovascular disorders, the importance of TMAO mediating inflammatory processes, and the potential utility of TMAO as a therapeutic target.
Xia C-L, Chu P, Liu Y-X, Qu X-L, Gao X-F, Wang Z-M, et al. ALDH2 rs671 polymorphism and the risk of heart failure with preserved ejection fraction (HFpEF) in patients with cardiovascular diseases. J Hum Hypertens. 2020;34:16–23. https://doi.org/10.1038/s41371-019-0182-2.
Guo F, Qiu X, Tan Z, Li Z, Ouyang D. Plasma trimethylamine n-oxide is associated with renal function in patients with heart failure with preserved ejection fraction. BMC Cardiovasc Disord. 2020;20:394. https://doi.org/10.1186/s12872-020-01669-w.
Hayashi T, Yamashita T, Watanabe H, Kami K, Yoshida N, Tabata T, et al. Gut microbiome and plasma microbiome-related metabolites in patients with decompensated and compensated heart failure. Circ J. 2018;83:182–92. https://doi.org/10.1253/circj.CJ-18-0468. This article demonstrated that plasma concentrations of trimethylamine-N-oxide (TMAO) were increased in heart failure patients, supporting the hypothesis that gut microbiome composition is altered in heart failure patients.
Salzano A, Israr MZ, Yazaki Y, Heaney LM, Kanagala P, Singh A, et al. Combined use of trimethylamine N-oxide with BNP for risk stratification in heart failure with preserved ejection fraction: findings from the DIAMONDHFpEF study. Eur J Prev Cardiol. 2020;27:2159–62. https://doi.org/10.1177/2047487319870355.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. https://doi.org/10.1101/cshperspect.a016295.
Arvidsson M, Ahmed A, Bouzina H, Rådegran G. Plasma proteoglycan prolargin in diagnosis and differentiation of pulmonary arterial hypertension. ESC Hear Fail. 2021;8:1230–43.
Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–9. https://doi.org/10.1038/nchembio.260.
Chirinos JA, Akers SR, Trieu L, Ischiropoulos H, Doulias P-T, Tariq A, et al. Heart failure, left ventricular remodeling, and circulating nitric oxide metabolites. J Am Heart Assoc. 2016;5:1–8.
Yu C-M, Fung PC-W, Chan G, Lai KW-H, Wang Q, Lau C-P. Plasma nitric oxide level in heart failure secondary to left ventricular diastolic dysfunction. Am J Cardiol. 2001;88:867–70. https://doi.org/10.1016/S0002-9149(01)01894-X.
Zamani P, French B, Brandimarto JA, Doulias P-T, Javaheri A, Chirinos JA, et al. Effect of heart failure with preserved ejection fraction on nitric oxide metabolites. Am J Cardiol. 2016;118:1855–60. https://doi.org/10.1016/j.amjcard.2016.08.077.
Szatmari T, Dobra K. The role of syndecan-1 in cellular signaling and its effects on heparan sulfate biosynthesis in mesenchymal tumors. Front Oncol. 2013;3:310. https://doi.org/10.3389/fonc.2013.00310. This article discussed the involvement of syndecans in the differentiation process across the epithelial-mesenchyma axis, mainly through their ability to bind to growth factors and modulate their downstream signaling.
Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech. 2000;50:184–95.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Chia YC, Kieneker LM, van Hassel G, Binnenmars SH, Nolte IM, van Zanden JJ, et al. Interleukin 6 and development of heart failure with preserved ejection fraction in the general population. J Am Heart Assoc. 2021;10:e018549. https://doi.org/10.1161/JAHA.120.018549.
Yan AT, Yan RT, Cushman M, Redheuil A, Tracy RP, Arnett DK, et al. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2010;31:875–82. https://doi.org/10.1093/eurheartj/ehp454. This article demonstrated that in asymptomatic men and women without documentedcardiovascular disease, there is a strong, independent, and inverse relationship between interleukin-6 (IL-6) and regional left ventricular systolic function. This suggests that IL-6 may underlie the pathogenetic link between inflammation, left ventricular dysfunction, and incipient heart failure.
Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, et al. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–95. https://doi.org/10.1093/eurjhf/hfr079.
Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci U S A. 1995;92:4862–6.
Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertens (Dallas, Tex 1979). 2010;56:225–31. https://doi.org/10.1161/HYPERTENSIONAHA.109.148635.
Wu C-K, Lee J-K, Chiang F-T, Yang C-H, Huang S-W, Hwang J-J, et al. Plasma levels of tumor necrosis factor-alpha and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit Care Med. 2011;39:984–92. https://doi.org/10.1097/CCM.0b013e31820a91b9.
Lakhani I, Wong MV, Hung JKF, Gong M, Bin WK, Xia Y, et al. Diagnostic and prognostic value of serum C-reactive protein in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart Fail Rev. 2021;26:1141–50. https://doi.org/10.1007/s10741-020-09927-x.
Rabkin S. Evaluating the adverse outcome of subtypes of heart failure with preserved ejection fraction defined by machine learning: a systematic review focused on defining high risk phenogroups. EXCLI J. 2022;21:487–518.
Rabkin SW, Langer A, Ur E, Calciu C-D, Leiter LA. Inflammatory biomarkers CRP, MCP-1, serum amyloid alpha and interleukin-18 in patients with HTN and dyslipidemia: impact of diabetes mellitus on metabolic syndrome and the effect of statin therapy. Hypertens Res. 2013;36:550–8. https://doi.org/10.1038/hr.2012.214.
Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ, Oude Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–59.
Kim Y-H, Nijst P, Kiefer K, Tang WHW. Endothelial glycocalyx as biomarker for cardiovascular diseases: mechanistic and clinical implications. Curr Heart Fail Rep. 2017;14:117–26. https://doi.org/10.1007/s11897-017-0320-5. Syndecan-1 (SDC-1) is a main constituent of endothelial glycocalyx. In patients with heart failure, elevated serum SDC-1 has been associated with worsening cardiac and renal function. This review article summarizes the existing literature on endothelial glycocalyx in cardiovascular diseases and their clinical implications.
Mamic P, Chaikijurajai T, Tang WHW. Gut microbiome - a potential mediator of pathogenesis in heart failure and its comorbidities: state-of-the-art review. J Mol Cell Cardiol. 2021;152:105–17. https://doi.org/10.1016/j.yjmcc.2020.12.001. This review article highlights the role that gut microbial metabolites (including short chain fatty acids, trimethylamine-N-oxide (TMAO), amino acid metabolites, and bile acids) play in heart failure pathophysiology and its potential as a novel therapeutic target in heart failure.
Zuo L, Chuang C-C, Hemmelgarn BT, Best TM. Heart failure with preserved ejection fraction: defining the function of ROS and NO. J Appl Physiol. 2015;119:944–51. https://doi.org/10.1152/japplphysiol.01149.2014.
Shea CM, Price GM, Liu G, Sarno R, Buys ES, Currie MG, et al. Soluble guanylate cyclase stimulator praliciguat attenuates inflammation, fibrosis, and end-organ damage in the Dahl model of cardiorenal failure. Am J Physiol Renal Physiol. 2020;318:F148–59. https://doi.org/10.1152/ajprenal.00247.2019. This article discusses how stimulate of soluble guanylate cyclase by praliciguat may be an effective mechanism for treating diseases linked to nitric oxide deficiency, particularly those associated with cardiac failure.
Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373:2314–24. https://doi.org/10.1056/NEJMoa1510774.
Chirinos JA, Londono-Hoyos F, Zamani P, Beraun M, Haines P, Vasim I, et al. Effects of organic and inorganic nitrate on aortic and carotid haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail. 2017;19:1507–15. https://doi.org/10.1002/ejhf.885.
Matyas C, Nemeth BT, Olah A, Torok M, Ruppert M, Kellermayer D, et al. Prevention of the development of heart failure with preserved ejection fraction by the phosphodiesterase-5A inhibitor vardenafil in rats with type 2 diabetes. Eur J Heart Fail. 2017;19:326–36. https://doi.org/10.1002/ejhf.711.
Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35. https://doi.org/10.1159/000338166. Inflammatory mediators are important in the pathogenesis of chronic heart failure contributing to cardiac remodeling and peripheral vascular disturbances. This review article argues for the possibility of future therapeutic targets such as mediators in innate immunity, chemokines, and mediators in matrix remodeling.
Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055–9.
Nouraei H, Rabkin SW. A new approach to the clinical subclassification of heart failure with preserved ejection fraction. Int J Cardiol. 2021;331:138–43. https://doi.org/10.1016/j.ijcard.2021.01.052.
Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–79. https://doi.org/10.1161/CIRCULATIONAHA.114.010637.
Segar MW, Patel KV, Ayers C, Basit M, Tang WHW, Willett D, et al. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur J Heart Fail. 2020;22:148–58. https://doi.org/10.1002/ejhf.1621.
Ge Z, Li A, McNamara J, Dos Remedios C, Lal S. Pathogenesis and pathophysiology of heart failure with reduced ejection fraction: translation to human studies. Heart Fail Rev. 2019;24:743–58. https://doi.org/10.1007/s10741-019-09806-0.
Author information
Authors and Affiliations
Contributions
XYG: Data curation, analysis, and writing.
SWR: Conceptualization, methodology, and writing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Clinical Heart Failure.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gui, X.Y., Rabkin, S.W. C-Reactive Protein, Interleukin-6, Trimethylamine-N-Oxide, Syndecan-1, Nitric Oxide, and Tumor Necrosis Factor Receptor-1 in Heart Failure with Preserved Versus Reduced Ejection Fraction: a Meta-Analysis. Curr Heart Fail Rep 20, 1–11 (2023). https://doi.org/10.1007/s11897-022-00584-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-022-00584-9