Abstract
Purpose of Review
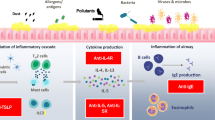
There is an emerging body of research on targeted biologic therapies for the treatment of severe inflammatory nasal disorders, especially chronic rhinosinusitis with nasal polyposis (CRSwNP). This paper will evaluate the efficacy of biologic therapies for severe nasal inflammation by summarizing key preclinical trials of biologics for animal models of allergic rhinitis and the recent phase 2 and 3 clinical trials of biologic therapies for CRSwNP.
Recent Findings
Biologics that target the IL-4 receptor (dupilumab), IgE (omalizumab), and IL-5 (mepolizumab, reslizumab, and benralizumab) in patients with CRSwNP have shown improvement of various metrics including Sino-Nasal Outcome Test (SNOT-22) scores, Nasal Polyp Scores (NPS), Nasal Congestion Scores (NCS), and Lund-Mackay sinus opacification scores.
Summary
The efficacy demonstrated through the dupilumab phase 3 trials (LIBERTY NP SINUS-24 and SINUS-52) led to approval of the first biologic for the treatment of CRSwNP. Phase 3 trials for omalizumab (POLYP 1 and 2) and mepolizumab (SYNAPSE study) and post hoc analyses of phase 3 asthma studies for reslizumab and benralizumab have also demonstrated positive results for the use of biologics for patients with CRSwNP. Future efficacy studies and risk/benefit and cost analyses of these biologics and other cytokine targets for allergic rhinitis with and without nasal polyposis need to be performed.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wheatley LM, Togias A. Clinical practice: allergic rhinitis. N Engl J Med. 2015;372(5):456–63.
Scadding G. Cytokine profiles in allergic rhinitis. Curr Allergy Asthma Rep. 2014;14:435.
Scadding GW, Calderon MA, Bellido V, Koed GK, Nielsen NC, Lund K, et al. Optimisation of grass pollen nasal allergen challenge for assessment of clinical and immunological outcomes. J Immunol Methods. 2012;384(1–2):25–32.
Benson M, Strannegard IL, Wennergren G, Strannegard O. Cytokines in nasal fluids from school children with seasonal allergic rhinitis. Pediatr Allergy Immunol. 1997;8(3):143–9.
Pilette C, Jacobson MR, Ratajczak C, Detry B, Banfield G, VanSnick J, et al. Aberrant dendritic cell function conditions Th2- cell polarization in allergic rhinitis. Allergy. 2013;68(3):312–21.
Gevaert P, Bruaene NV, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–95.
Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41.
Nicholson GC, Kariyawasam HH, Tan AJ, Hohfeld JM, Quinn D, Walker C, et al. The effects of an anti-IL-13 mAb on cytokine levels and nasal symptoms following nasal allergen challenge. J Allergy Clin Immunol. 2011;128:800–7.
Miyahara S, Miyahara N, Matsubara S, Takeda K, Koya T, Gelfand EW. IL-13 is essential to the late-phase response in allergic rhinitis. J Allergy Clin Immunol. 2006;118:1110–6.
Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012;130:829–42.
Wang Y, McCusker CT. Interleukin-13-dependent bronchial hyper-responsiveness following isolated airway allergen challenge in a murine model of allergic rhinitis and asthma. Clin Exp Allergy. 2005;35:1104–11.
Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365(12):1088–98.
Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988;168(3):853–62.
Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–66.
Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10(10):493–9.
Bachert C, Mannent L, Naclerio R, Mullol J, Ferguson B, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469–79.
Swanson BN, Mannent L, Hamilton JD, Zhang D, Tian N, Wang Y, et al. The effect of dupilumab on biomarkers in the peripheral blood and nasal secretions in the treatment of chronic sinusitis with nasal polyposis. Inflamm Res. 2015;64(2 Suppl 1):S118–9.
Jonstam K, Swanson BN, Mannent LP, Cardell LO, Tian N, Wang Y, et al. Dupilumab reduces local Type-2 proinflammatory biomarkers in patients with chronic sinusitis and nasal polyposis. Allergy. 2019;74(4):743–52.
•• Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–50 Dupilumab phase 3 trials demonstrating efficacy and safety, which led to approval of dupilumab as the first biologic for the treatment of CRSwNP.
Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013 Sep;131(1):110–6.
•• Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020;146(3):595–605 Omalizumab phase 3 trials demonstrating efficacy and safety for patients with CRSwNP.
Corren J. Anti-interleukin-5 antibody therapy in asthma and allergies. Curr Opin Allergy Clin Immunol. 2011;11(6):55–570.
Corren J. Inhibition of interleukin-5 for the treatment of eosinophilic diseases. Discov Med. 2012;13(71):305–12.
Asakura K, Saito H, Watanabe M, Ogasawara H, Matsui T, Kataura A. Effects of anti-IL-5 monoclonal antibody on the murine model of nasal allergy. Int Arch Allergy Immunol. 1998;116(1):49–52.
Saito H, Matsumoto K, Denburg AE, Crawford L, Ellis R, Inman MD, et al. Pathogenesis of murine experimental allergic rhinitis: a study of local and systemic consequences of IL-5 deficiency. J Immunol. 2002;168(6):3017–23.
• Weinstein SF, Katial RK, Bardin P, Korn S, McDonald M, Garin M, et al. Effects of reslizumab on asthma outcomes in a subgroup of eosinophilic asthma patients with self-reported chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2019;7(2):589–96 Study showing that patients with CRSwNP had a larger reduction in frequency of asthma attacks compared to asthma patients without CRSwNP when treated with reslizumab.
• Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. 2017;140(4):1024–31 Study showing decreased need for nasal surgery in patients with CRSwNP treated with mepolizumab.
•• Hopkins C, Bachert C, Fokkens W, Desrosiers M, Wagenmann M, Lee SE, et al. Add-on mepolizumab for chronic rhinosinusitis with nasal polyps: SYNAPSE study. Poster presented at the International Congress of the European Respiratory Society, September 7-9, 2020. Abstract in European Respiratory Journal. 2020;56:4616. Mepolizumab phase 3 study demonstrating efficacy and safety for patients with CRSwNP.
Lombardo N, Pelaia C, Ciriolo M, Della Corte M, Piazzetta G, Lobello N, et al. Real-life effects of benralizumab on allergic chronic rhinosinusitis and nasal polyposis associated with severe asthma. Int J Immunopathol Pharmacol. 2020;34:1–8.
• Maspero J, Harrison T, Werkstrom V, Wu Y, Gopalan G, Zangrilli J. Clinical efficacy of benralizumab in patients with severe uncontrolled eosinophilic asthma and nasal polyposis: pooled analysis of the SIRCCO and CALIMA trials. Abstract in J Allergy Clin Immunol. 2018 1;141(2):AB12. Study showing a larger reduction of asthma exacerbations in patients with nasal polyps treated with benralizumab.
• Harrison TW, Chanez P, Menzella F, Walter Canonica G, Louis R, Cosio BG, et al. Exacerbation reduction and early and sustained improvements in SGRQ, lung function, and symptoms of nasal polyposis with benralizumab for severe, eosinophilic asthma: phase IIIb ANDHI Trial. Poster presented at the American Thoracic Society International Conference, May 15-20, 2020. Study showing improvement in sino-nasal symptoms in patients with CRSwNP treated with benralizumab.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98.
Wang M, Zhang W, Shang J, Yang J, Zhang L, Bachert C. Immunomodulatory effects of IL-23 and IL-17 in a mouse model of allergic rhinitis. Clin Exp Allergy. 2013;43(8):956–66.
Kim YH, Yang TY, Park CS, Ahn SH, Son BK, Kim JH, et al. Anti-IL-33 antibody has a therapeutic effect in a murine model of allergic rhinitis. Allergy. 2012;67(2):183–90.
Song W, Wang C, Zhou J, Pan S, Lin S. IL-33 expression in chronic rhinosinusitis with nasal polyps and its relationship with clinical severity. ORL J Otorhinolaryngol Relat Spec. 2017;79(6):323–30.
Mulroy D. Anaptysbio reports top-line data from interim analysis of ECLIPSE phase 2 clinical trial of etokimab in chronic rhinosinusitis with nasal polyps. AnaptysBio Website. Accessed 2 Dec 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author BG has the following disclosures relevant to this manuscript: speaking honoraria from GSK, Sanofi, Regeneron, and OptiNose as well as consulting fees from Regeneron and Astra-Zeneca; Research Support from Genentech.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rhinitis, Conjunctivitis, and Sinusitis
Rights and permissions
About this article
Cite this article
Geng, B., Dilley, M. & Anterasian, C. Biologic Therapies for Allergic Rhinitis and Nasal Polyposis. Curr Allergy Asthma Rep 21, 36 (2021). https://doi.org/10.1007/s11882-021-01013-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11882-021-01013-y