Abstract
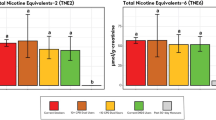
This study assessed changes in biomarkers of exposure (BoE) after 5 days of completely or partially switching to an electronic nicotine delivery system (ENDS) use, compared with continued use of combustible cigarettes and smoking abstinence among Chinese adult smokers. A randomized, open-label, parallel-arm study was conducted among Chinese adult smokers who were naive ENDS users. Forty-six subjects were randomized to 4 study groups (n = 11–12 per group): exclusive ENDS use, dual use of ENDS and cigarettes, exclusive cigarettes use, and smoking abstinence. Subjects were confined in clinic for 5 consecutive days and product use was ad libitum. Nicotine and its metabolites (cotinine and 3-hydroxycotinine), and BoEs (AAMA, CEMA, HEMA, HMPMA, 3-HPMA, SPMA, exhaled CO, and exhaled NO) were measured. Withdrawal symptom was measured using MNWS throughout the 5-day period. Six urine BoEs of volatile organic compounds decreased by 55.1–84.1% in the exclusive ENDS use group, which is similar to the smoking abstinence group (67.2–87.4%). The level of decrease was 56.8–70.4% in the dual use group and 10.7–39.0% in the cigarettes group. Urine total nicotine exposure had a non-significant increase in the exclusive ENDS use group, and plasma nicotine and cotinine showed a trend of increasing day by day. After completely or partially switching to ENDS use among Chinese smokers, exposure to selected toxicants were significantly decreased. The results of this study add to the body of evidence that exposure to toxic substance decreased among smokers after complete or partial switch from combustible cigarettes to ENDS use. As part of transition to experienced ENDS use, this study found that smokers of the initial stage who have no prior ENDS experience may increase nicotine intake after switching to ENDS use.

Similar content being viewed by others
Data availability
Data are available on reasonable request to the corresponding author.
References
Wald NJ, Hackshaw AK (1996) Cigarette smoking: an epidemiological overview. Br Med Bull 52(1):3–11. https://doi.org/10.1093/oxfordjournals.bmb.a011530
National Health Commission of the People’s Republic of China (2021) 2020 Report on Health Hazards of Smoking in China. Beijing, National Health Commision of the People’s Republic of China.
Institute of Medicine Committee to Assess the Science Base for Tobacco Harm R (2001) Clearing the smoke: assessing the science base for tobacco harm reduction. In: Stratton K, Shetty P, Wallace R, Bondurant S (eds) Washington (DC), National Academies Press (US) Copyright 2001 by the National Academy of Sciences. All rights reserved.
Hatsukami DK, Carroll DM (2020) Tobacco harm reduction: Past history, current controversies and a proposed approach for the future. Prev Med 140:106099. https://doi.org/10.1016/j.ypmed.2020.106099
National Academies of Sciences E, Medicine, Health, Medicine D, Board on Population H, Public Health PCommittee on the Review of the Health Effects of Electronic Nicotine Delivery S (2018) Public Health Consequences of E-Cigarettes. Eaton DL, Kwan LY, Stratton K (eds) Washington (DC), National Academies Press (US), Copyright 2018 by the National Academy of Sciences. All rights reserved
Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H (2014) Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction 109(11):1801–1810. https://doi.org/10.1111/add.12659
Tayyarah R, Long GA (2014) Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol 70(3):704–710. https://doi.org/10.1016/j.yrtph.2014.10.010
Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N (2014) Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23(2):133–139. https://doi.org/10.1136/tobaccocontrol-2012-050859
Geiss O, Bianchi I, Barahona F, Barrero-Moreno J (2015) Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int J Hyg Environ Health 218(1):169–180. https://doi.org/10.1016/j.ijheh.2014.10.001
Blair SL, Epstein SA, Nizkorodov SA, Staimer N (2015) A real-time fast-flow tube study of VOC and particulate emissions from electronic, potentially reduced-harm, conventional, and reference cigarettes. Aerosol Sci Technol 49(9):816–827. https://doi.org/10.1080/02786826.2015.1076156
Kanobe MN, Jones BA, Nelson P, Brown BG, Chen P, Makena P, Schmidt E, Darnell J, Caraway JW, Prasad GL, Nordskog B, Round EK (2022) Part three: a randomized study to assess biomarker changes in cigarette smokers switched to Vuse Solo or Abstinence. Sci Rep 12(1):20658. https://doi.org/10.1038/s41598-022-25054-z
Round EK, Chen P, Taylor AK, Schmidt E (2019) Biomarkers of tobacco exposure decrease after smokers switch to an E-cigarette or nicotine gum. Nicotine Tob Res 21(9):1239–1247. https://doi.org/10.1093/ntr/nty140
O’Connell G, Graff DW, D’Ruiz CD (2016) Reductions in biomarkers of exposure (BoE) to harmful or potentially harmful constituents (HPHCs) following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Toxicol Mech Methods 26(6):443–454. https://doi.org/10.1080/15376516.2016.1196282
Jay J, Pfaunmiller EL, Huang NJ, Cohen G, Graff DW (2020) Five-day changes in biomarkers of exposure among adult smokers after completely switching from combustible cigarettes to a nicotine-salt pod system. Nicotine Tob Res 22(8):1285–1293. https://doi.org/10.1093/ntr/ntz206
Cohen G, Goldenson NI, Bailey PC, Chan S, Shiffman S (2021) Changes in biomarkers of cigarette smoke exposure after 6 days of switching exclusively or partially to use of the JUUL system with two nicotine concentrations: a randomized controlled confinement study in adult smokers. Nicotine Tob Res 23(12):2153–2161. https://doi.org/10.1093/ntr/ntab134
Morris P, McDermott S, Chapman F, Verron T, Cahours X, Stevenson M, Thompson J, Chaudhary N, O’Connell G (2022) Reductions in biomarkers of exposure to selected harmful and potentially harmful constituents following exclusive and partial switching from combustible cigarettes to myblu(™) electronic nicotine delivery systems (ENDS). Intern Emerg Med 17(2):397–410. https://doi.org/10.1007/s11739-021-02813-w
George J, Hussain M, Vadiveloo T, Ireland S, Hopkinson P, Struthers AD, Donnan PT, Khan F, Lang CC (2019) Cardiovascular effects of switching from tobacco cigarettes to electronic cigarettes. J Am Coll Cardiol 74(25):3112–3120. https://doi.org/10.1016/j.jacc.2019.09.067
Polosa R, Morjaria JB, Prosperini U, Busà B, Pennisi A, Malerba M, Maglia M, Caponnetto P (2020) COPD smokers who switched to e-cigarettes: health outcomes at 5-year follow up. Ther Adv Chronic Dis 11:2040622320961617. https://doi.org/10.1177/2040622320961617
Hukkanen J, Jacob P 3rd, Benowitz NL (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57(1):79–115. https://doi.org/10.1124/pr.57.1.3
Neafsey P, Ginsberg G, Hattis D, Johns DO, Guyton KZ, Sonawane B (2009) Genetic polymorphism in CYP2E1: Population distribution of CYP2E1 activity. J Toxicol Environ Health B Crit Rev 12(5–6):362–388. https://doi.org/10.1080/10937400903158359
Xu T, Niu ZY, Xu J, Li XD, Luo Q, Luo A, Huang YL, Jiang XT, Wu ZH (2022) Chemical analysis of selected harmful and potentially harmful constituents and in vitro toxicological evaluation of leading flavoured e-cigarette aerosols in the Chinese market. Drug Test Anal. https://doi.org/10.1002/dta.3337
Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P 3rd, Benowitz NL (2017) Exposure to Nicotine and Selected Toxicants in Cigarette Smokers Who Switched to Electronic Cigarettes: A Longitudinal Within-Subjects Observational Study. Nicotine Tob Res 19(2):160–167. https://doi.org/10.1093/ntr/ntw160
Yu X, Xiao D, Li B, Liu Y, Wang G, Chen J, Bai C, Pan J, Wan H, Li Q, Zhou X, Liao R, Li Q, Wang C, Chen R, Tang Y, Mo H, Zhao M, Du J, Li J, Kang L, Wang C (2010) Evaluation of the Chinese versions of the Minnesota nicotine withdrawal scale and the questionnaire on smoking urges-brief. Nicotine Tob Res 12(6):630–634. https://doi.org/10.1093/ntr/ntq063
Zhang G, Zhan J, Fu H (2022) Trends in smoking prevalence and intensity between 2010 and 2018: implications for tobacco control in China. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph19020670
D’Ruiz CD, Graff DW, Robinson E (2016) Reductions in biomarkers of exposure, impacts on smoking urge and assessment of product use and tolerability in adult smokers following partial or complete substitution of cigarettes with electronic cigarettes. BMC Public Health 16:543. https://doi.org/10.1186/s12889-016-3236-1
Benowitz NL, St Helen G, Nardone N, Cox LS, Jacob P (2020) Urine metabolites for estimating daily intake of nicotine from cigarette smoking. Nicotine Tob Res 22(2):288–292. https://doi.org/10.1093/ntr/ntz034
Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, Kyrzopoulos S, Poulas K, Voudris V (2015) Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers). Sci Rep 5:11269. https://doi.org/10.1038/srep11269
Goempel K, Tedsen L, Ruenz M, Bakuradze T, Schipp D, Galan J, Eisenbrand G, Richling E (2017) Biomarker monitoring of controlled dietary acrylamide exposure indicates consistent human endogenous background. Arch Toxicol 91(11):3551–3560. https://doi.org/10.1007/s00204-017-1990-1
Erkekoglu P, Baydar T (2014) Acrylamide neurotoxicity. Nutr Neurosci 17(2):49–57. https://doi.org/10.1179/1476830513y.0000000065
Koszucka A, Nowak A, Nowak I, Motyl I (2020) Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit Rev Food Sci Nutr 60(10):1677–1692. https://doi.org/10.1080/10408398.2019.1588222
Keith RJ, Fetterman JL, Orimoloye OA, Dardari Z, Lorkiewicz PK, Hamburg NM, DeFilippis AP, Blaha MJ, Bhatnagar A (2020) Characterization of volatile organic compound metabolites in cigarette smokers, electronic nicotine device users, dual users, and nonusers of tobacco. Nicotine Tob Res 22(2):264–272. https://doi.org/10.1093/ntr/ntz021
Rubinstein ML, Delucchi K, Benowitz NL, Ramo DE (2018) Adolescent exposure to toxic volatile organic chemicals from E-cigarettes. Pediatrics. https://doi.org/10.1542/peds.2017-3557
Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, Wang L, Christensen C, Ambrose B, Borek N, van Bemmel D, Konkel K, Erives G, Stanton CA, Lambert E, Kimmel HL, Hatsukami D, Hecht SS, Niaura RS, Travers M, Lawrence C, Hyland AJ (2018) Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open 1(8):e185937. https://doi.org/10.1001/jamanetworkopen.2018.5937
Lorkiewicz P, Riggs DW, Keith RJ, Conklin DJ, Xie Z, Sutaria S, Lynch B, Srivastava S, Bhatnagar A (2019) Comparison of urinary biomarkers of exposure in humans using electronic cigarettes, combustible cigarettes, and smokeless tobacco. Nicotine Tob Res 21(9):1228–1238. https://doi.org/10.1093/ntr/nty089
Henning RJ, Johnson GT, Coyle JP, Harbison RD (2017) Acrolein can cause cardiovascular disease: a review. Cardiovasc Toxicol 17(3):227–236. https://doi.org/10.1007/s12012-016-9396-5
Bein K, Leikauf GD (2011) Acrolein—a pulmonary hazard. Mol Nutr Food Res 55(9):1342–1360. https://doi.org/10.1002/mnfr.201100279
IARC (2007) 1, 3-butadiene, ethylene oxide and vinyl halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide)
Todd G, Faroon O, Jones D, Lumpkin M, Stickney J, Citra M (2006) Toxicological profile for vinyl chloride. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles, Atlanta
Coenraads PJ, Bleumink E, Nater JP (1975) Susceptibility to primary irritants: age dependence and relation to contact allergic reactions. Contact Dermatitis 1(6):377–381. https://doi.org/10.1111/j.1600-0536.1975.tb05478.x
Wang L, Yang Z, Xu L, Pan X, Liu X, Zhao J, Li X, Zhu M, Xie J (2018) Acute exposure to crotonaldehyde induces dysfunction of immune system in male Wistar rats. J Toxicol Sci 43(1):33–44. https://doi.org/10.2131/jts.43.33
Snyder R (2012) Leukemia and benzene. Int J Environ Res Public Health 9(8):2875–2893. https://doi.org/10.3390/ijerph9082875
Czoli CD, Fong GT, Goniewicz ML, Hammond D (2019) Biomarkers of exposure among “Dual Users” of tobacco cigarettes and electronic cigarettes in Canada. Nicotine Tob Res 21(9):1259–1266. https://doi.org/10.1093/ntr/nty174
Smith DM, Christensen C, van Bemmel D, Borek N, Ambrose B, Erives G, Niaura R, Edwards KC, Stanton CA, Blount BC, Wang L, Feng J, Jarrett JM, Ward CD, Hatsukami D, Hecht SS, Kimmel HL, Travers M, Hyland A, Goniewicz ML (2021) Exposure to nicotine and toxicants among dual users of tobacco cigarettes and E-cigarettes: Population Assessment of Tobacco and Health (PATH) Study, 2013–2014. Nicotine Tob Res 23(5):790–797. https://doi.org/10.1093/ntr/ntaa252
Lizhnyak PN, Noggle B, Wei L, Edmiston J, Becker E, Black RA, Sarkar M (2022) Understanding heterogeneity among individuals who smoke cigarettes and vape: assessment of biomarkers of exposure and potential harm among subpopulations from the PATH Wave 1 Data. Harm Reduct J 19(1):90. https://doi.org/10.1186/s12954-022-00673-x
Guo Y, Li S, Wang Z, Jiang F, Guan Y, Huang M, Zhong G (2022) Nicotine delivery and pharmacokinetics of an electronic cigarette compared with conventional cigarettes in Chinese adult smokers: a randomized open-label crossover clinical study. Nicotine Tob Res 24(12):1881–1888. https://doi.org/10.1093/ntr/ntac143
Hartmann-Boyce J, Lindson N, Butler AR, McRobbie H, Bullen C, Begh R, Theodoulou A, Notley C, Rigotti NA, Turner T, Fanshawe TR, Hajek P (2022) Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 11(11):Cd010216. https://doi.org/10.1002/14651858.CD010216.pub7
Benowitz NL (2010) Nicotine addiction. N Engl J Med 362(24):2295–2303. https://doi.org/10.1056/NEJMra0809890
Rose JE (2006) Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology 184(3–4):274–285. https://doi.org/10.1007/s00213-005-0250-x
Chen Z, Peto R, Zhou M, Iona A, Smith M, Yang L, Guo Y, Chen Y, Bian Z, Lancaster G, Sherliker P, Pang S, Wang H, Su H, Wu M, Wu X, Chen J, Collins R, Li L (2015) Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet 386(10002):1447–1456. https://doi.org/10.1016/s0140-6736(15)00340-2
Acknowledgements
The authors thank the medical team in the Clinical Trial Center of Dongguan Kanghua Hospital who participated in the conduction and performance of this study. The authors also thank the cooperation and support of Guangzhou Determine Biotech Co, Ltd, Guangdong Huawei Testing Co, Ltd, and Guangzhou Huaqi Yixin Technology Co, Ltd.
Funding
This study was supported and funded by RELX Lab, Shenzhen RELX Tech. Co, Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by RELX Tech. CO, Ltd. At the time of the research, Kun Duan, Zehong Wu and Xingtao Jiang were employees of RELX Tech. Other authors declared no other conflict of interest.
Ethical approval
This study protocol and informed consent form were reviewed and approved by the Clinical Trial Research Ethics Committee of Dongguan Kanghua Hospital (Dongguan, China) (Approved No. of ethic committee: 20200701-KHCTEC-SCYJTZ-V04).
Informed consent
All participants provided written informed consent before enrolment into the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The figures 1, 2, 3 and the graphical abstract were published with incomplete graphics and low resolution. The corrected figures with high resolution were updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Guo, Y., Duan, K. et al. Changes in biomarkers of exposure and withdrawal symptom among Chinese adult smokers after completely or partially switching from combustible cigarettes to an electronic nicotine delivery system. Intern Emerg Med 19, 669–679 (2024). https://doi.org/10.1007/s11739-023-03518-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-023-03518-y