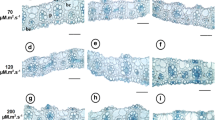
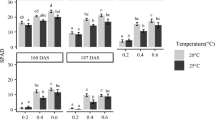
Abstract
Growth and photosynthetic capacity of Cattleya warneri T. Moore seedlings cultivated in vitro were evaluated in two environments: (1) growth room (GR) with constant light, humidity, and temperature; and (2) greenhouse (GH) with variable humidity, temperature, and light intensity and quality. In both environments, two different tissue culture vessel lids were used: transparent plastic lids (TCVplastic) and non-transparent metal lids (TCVmetal). After 11 months of in vitro cultivation, five seedlings from each tissue culture vessel were evaluated for growth and photosynthetic capacity, while the other five seedlings of each tissue culture vessel were transferred to a greenhouse for acclimatization. Increased biomass production in vitro was observed in GH and GR (GH>GR). However, the photosynthetic capacity was not altered by the GH environment, since the net photosynthetic rate at 300 μmol m−2 s−1 (NPR300) was low in the final of period in all treatments. Therefore, the increase in biomass production in C. warneri was mostly dependent on the exogenous carbon source through the addition of sucrose to the culture medium. The use of TCVplastic in vitro improved seedlings’ growth in both GR and GH, showing an advantage in relation to TCVmetal. GH environment with quality light spectrum and thermal amplitude, and use of TCVplastic, increased biomass production in vitro and improved the acclimatization process of C. warneri seedlings, which also reduced electricity costs since the use of artificial light and air conditioning is not required.








Similar content being viewed by others
Abbreviations
- Fv/Fm :
-
Maximum quantum yield of photosystem II;
- GH:
-
Greenhouse;
- GR:
-
Growth room;
- IVH:
-
In vitro hardening;
- PAR:
-
Photosynthetically active radiation;
- DLI:
-
Daily light integral;
- PI:
-
Photosynthetic index;
- RDW:
-
Root dry weight;
- RFW:
-
Root fresh weight;
- RH:
-
Relative humidity;
- SDW:
-
Shoot dry weight;
- SFW:
-
Shoot fresh weight;
- Tair:
-
Air temperature;
- TCVmetal :
-
Tissue culture vessel with non-transparent metal lids;
- TCVplastic :
-
Tissue culture vessel with transparent plastic lids;
- TCV:
-
Tissue culture vessel;
- TDW:
-
Total dry weight;
- TFW:
-
Total fresh weight;
- VPDair :
-
Air vapor pressure deficit
References
Abreu PP, Souza MM, Almeida AAF, Santos EA, Freitas JCO, Figueiredo AL (2014) Photosynthetic responses of ornamental passion flower hybrids to varying light intensities. Acta Physiol Plant 36:1993–2004
Batista DS, Felipe SHS, Silva TD, De Castro KM, Mamedes-Rodrigues TC, Miranda NA, Ríos-Ríos AM, Faria DV, Fortini EA, Chagas K, Torres-Silva G, Xavier A, Arencibia AD, Otoni WC (2018) Light quality in plant tissue culture: does it matter? In Vitro Cell Dev Biol - Plant 54:195–215
Bennett SM, Tibbitts TW, Cao W (1991) Diurnal temperature fluctuation effects on potatoes grown with 12 h photoperiod. Am Potato J 68:81–86
Bohlar-Nordenkampf HR, Draxler G (1993) Functional leaf anatomy. In: Hall DO, Scurlock JMO, Bohlar-Nordenkampf HR, Leegood LC, Long SP (eds) Photosynthesis and productioan in a changing environmental: a field and laboratory manual. Chapman & Hall, London, pp 91–112
Borges D, Oliveira MC, Penoni ES, Pádua TRP, Braga FT, Pasqual M (2011) Microprogation of chrysanthemum (Dendranthema grandiflora tzevele cv. rage) under natural and artificial light in different concentration of the culture media. Plant Cell Cult Microprop 7:1–8
Cassells AC, Walsh C (1994) The influence of gas permeability of the culture lid on calcium uptake and stomatal function in Dianthus microplants. Plant Cell Tiss Org Cult 37:171–178
Cerasoli S, Wertin T, Mcguire MA, Rodrigues A, Aubrey DP, Pereira JS, Teskey RO (2014) Poplar saplings exposed to recurring temperature shifts of different amplitude exhibit differences in leaf gas exchange and growth despite equal mean temperature. AoBP 6:1–9
Chen C (2006) In situ measurement of microclimate for the plantlets cultured in vitro. Biosyst Eng 95:413–423
De Riek J, Piqueras A, Deberegh PC (1997) Sucrose uptake and metabolism in a double layer system for micropropagation of Rosa multiflora. Plant Cell Tiss Org Cult 47:269–278
Demmig B, Björkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 171:171–184
Deng R, Donnelly DJ (1993) In vitro hardening of red raspberry through CO2 enrichment and relative humidity reduction on sugar-free medium. Can J Plant Sci 73:1105–1113
Erig AC, Schuch MW (2005) Micropropagação fotoautotrófica e uso da luz natural. Cienc Rural 35:961–965
Ferreira WM, Suzuki RM (2008) O cultivo in vitro de orquídeas como alternativa para a preservação de espécies nativas ameaçadas de extinção. In: Loiola MIB, Baseia IG, Lichston JE (eds) Atualidades, desafios e perspectiva da botânica no Brasil. Imagem Gráfica, Natal, pp 67–68
Fini A, Loreto F, Tattini M, Giordano C, Ferrini F, Brunetti C, Cetritto M (2016) Mesophyll conductance plays a central role in leaf functioning of Oleaceae species exposed to contrasting sunlight irradiance. Physiol Plant 157:54–68
Fuentes G, Talavera C, Desjardins Y, Santamaría JM (2007) Low exogenous sucrose improves ex vitro growth and photosynthesis in coconut in vitro plantlets if grown in vitro under high light. Acta Hort 748:151–156
Fujiwara K, Kozai T (1995) Physical microenvironment and its effects. In: Aitken-Christie J, Kozai T, Smith MAL (eds) Automation and environmental control in plant tissue culture. Springer, Dordrecht, pp 319–369
Genty B, Goulas Y, Dimon B, Peltier JM, Moya I (1992) Modulation of efficiency of primary conversion in leaves, mechanisms involved at PSII. In: Murata N (ed) Research in Photosynthesis. Volume 4. Dordrecht, Kluwer Academic Publishers, pp 603–10
Gilliham M, Dayod M, Hocking BJ, Xu B, Conn SJ, Kaiser BN, Leigh RA, Tyerman SD (2011) Calcium delivery and storage in plant leaves: exploring the link with water flow. J Exp Bot 62:2233–2250
Hdider C, Desjardins Y (1994) Effects of sucrose on photosynthesis and phosphoenol pyruvate carboxylase activity of in vitro cultured strawberry plantlets. Plant Cell Tiss Org Cult 36:27–36
Heins J, Welker P, Schonlein C, Born I, Hardtrodt B, Neubert K, Tsuru D, Barth A (1988) Mechanism of proline-specific proteinases: (I) substrate specificity of dipeptidyl peptidase IV from pig kidney and proline-specific endopeptidase from Flavobacterium meningosepticurn. Biochim Biophys Acta 954:161–169
Jeong BR, Fujiwara K, Kozai T (1995) Environmental control and photoautotrophic micropropagation. Hort Rev 17:826–873
Jones HG (1992) Plant and microclimate: a quantitative approach to environmental plant physiology. Cambridge University Press, Cambridge, p 456
Kodym A, Zapata-Arias FJ (1999) Natural light as an alternative light source for the in vitro culture of banana (Musa acuminata cv. ‗’Grande Naine’). Plant Cell Tiss Org Cult 55:141–145
Kozai T (1991) Photoautotrophic micropropagation. In Vitro Cell Dev Biol - Plant 27:47–51
Kozai T, Kubota C, Jeong BR (1997) Environmental control for the large-scale production of plants through in vitro techniques. Plant Cell Tiss Org Cult 51:49–56
Kozai T, Kushihashi S, Kubota C, Fujiwara K (1992) Effect of the difference between photoperiod and dark period temperatures, and photosynthetic photon flux density on the shoot length and growth of potato plantlets in vitro. J Jpn Soc Hort Sci 61:93–98
Kozai T, Smith MAL (1995) Environmental control in plant tissue culture — general introduction and overview. In: Aitken-Christie J, Kozai T, Smith MAL (eds) Automation and environmental control in plant tissue culture. Springer, Dordrecht, pp 301–318
Kuhn BC, Claudino LO, Kuhn SB, Gutierre MAMG, Mangolin CA, Machado MFPSM (2014) Micropropagation of Cattleya forbesii Lindley (Orchidaceae) using combinations of auxin and cytokinin. Pleiade 8:83–82
Lambers H, Stuart Chapin III F, Pons TL (2008) Plant physiological ecology. Dordrecht, Springer, p 604
Li C-X, Xu Z-G, Dong R-Q, Chang S-X, Wang L-Z, Khalil-Ur-Rehman M, Tao J-M (2017) An RNA-Seq analysis of grape plantlets grown in vitro reveals different responses to blue, green, red led light, and white fluorescent light. Front Plant Sci 8:78
Li T, Yang Q (2015) Advantages of diffuse light for horticultural production and perspectives for further research. Front Plant Sci 6:1–6
Loveys BR, Scheurwater I, Pons TL, Fitter AH, Atkin OK (2002) Growth temperature influences the underlying components of relative growth rate: an investigation using inherently fast- and slow-growing plant species. Plant Cell Environ 25:975–988
Mano, J. (2002). Early events in environmental stresses in plants induction mechanisms of oxidative stress. In: Inze D, Montganu MV (eds) Oxidative Stress in Plants. Taylor & Francis, pp 217–246
Matthys D, Gielis J, Debergh P (1995) Ethylene. In: Aitken-Christi J, Kozai M, Simith LMA (eds) Automation and environmental control in plant tissue culture. Springer, Dordrecht, pp 473–491
Maxwell K, Johnson G N (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Mazzanatti T, Calzavara AK, Pimenta JA, Oliveira HC, Stolf-Moreira R, Bianchini E (2016) Light acclimation in nursery: morphoanatomy and ecophysiology of seedlings of three light-demanding neotropical tree species. Braz J Bot 39:19–28
Moe R, Heins RD, Erwin J (1991) Stem elongation and flowering of the long-day plant Campanula isophylla ‘Moretti’ in response to day and night temperature alternations and light quality. Sci Hort 48:141–151
Mothé GPB, Netto AT, Crespo LEC, Campostrini E (2008) Photochemical efficiency and growth characteristics of sugarcane (Saccharum officinarum L.) grown in vitro at different concentrations of sucrose and light quality. Plant Cell Cult Microprop 4:84–91
Nguyen QT, Kozai T (2005) Photoautotrophic micropropagation of woody species. In: Kozai T, Afreen F, Zobayed SMA (eds) Photoautotrophic (sugar-free medium) micropropagation as a new micropropagation and transplant production system. Springer, Dordrecht, pp 123–146
Otroshy M, Zamani A, Ebrahimi M, Struik PC (2009) Effect of exogenous hormones and chilling on dormancy breaking of seeds of Asafoetida (Ferula assafoetida L.). Res J Seed Sci 2:9–15
Pedmale UV, Huang SC, Zander M, Cole BJ, Hetzel J, Ljung K, Chory J (2016) Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 164:233–245
Perez Martinez LV, Melgarejo LM (2015) Photosynthetic performance and leaf water potential of gulupa (Passiflora edulis sims, passifloraceae) in the reproductive phase in three locations in the colombian andes. Acta Biolo Colomb 20:183–194
Pospisilová J, Solarová, Catsky J (1992) Photosynthetic responses to stress during in vitro cultivation. Photosynthetica 26:3–18
Preece JE, Sutter E (1991) Acclimatization of microprop Mi cropropagation: technology and applicationagated plants to the greenhouse and field. In: Debergh PC, Zimmerman (eds) . Kluwer Academic Publishers, Dordrecht, pp 71–94
Rezende RKS, Paiva LV, Paiva R, Chalfun Júnior A, Torga PP, Castro EM (2008) Organogenesis in floral chapters and evaluation of anatomical characteristics of the leaf of Gerbera jamesonni Adlam. Sci Agrotec 32:821–827
SAEG (2007) System for Statistical Analysis, Version 9.1 Arthur Bernardes Foundation - Federal University of Viçosa, Viçosa – MG.
Schmildt O, Torres-Netto A, Schmildt ER, Carvalho VS, Otoni WC, Campostrini E (2015) Photosynthetic capacity, growth, and water relations in Golden papaya cultivated in vitro with modifications in light quality, sucrose concentration and ventilation. Theor Exp Plant Physiol 27:7–18
Solarova J, Souckova D, Ullmann J, Pospisilova J (1996) In vitro culture: environmental conditions and plantlet growth as affected by vessel and stopper types. Hort Sci 23:51–58
Stancato GC, Bemelmans PF, Vegro CCLR (2001) Production of orchid seedlings from in vitro seeds and their economic viability: a case study. Ornam Hort 7:25–33
Stancato GC, Tucci MLSA (2010) Monitoring the end of the in vitro phase of Anthurium andreanum Lindl. Plantlets. Brazilian J Plant Physiol 22:61–68
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP test. In: Mathis P (ed) Photosynthesis: From Light to Biosphere. Kluwer Academic Publishers, the Netherlands, pp 977–980
Strasser RJ, Srivastava A, Tsimilli-Michael M (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou G, Govindjee (eds) Advances in Photosynthesis and Respiration. Chlorophyll fluorescence: a Signature of photosynthesis. Kluwer Academic Publishers, the Netherlands, pp 321–362
Strasser RJ, Tsimilli-Michael M, Srivastava A (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pather U, Mohanly P (eds) Probing Photosynthesis: Mechanisms. Regulation and Adaptation. Taylor and Francis, London, pp 445–483
Watanabe Y, Yamaguchi M, Sakamoto J, Tamai Y (1993) Characterization of plasma membrane H+-ATPase from salt-tolerant yeast Candida versatilis. Yeast 9:213–220
Wellburn AR (1994) The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144(3):307–313
Witkowski ETF, Lamont BB (1991) Leaf specific mass confounds leaf density and thickness. Oecologia 88:486–493
Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. Plant Cell Tiss Org Cult 105:149–158
Zhang S, Ma K, Chen L (2003) Response of photosynthetic plasticity of Paeonia suffruticosa to changed light environments. Environ Exp Bot 49:121–133
Ziv M (1995) In vitro acclimatization. In: Aitken-Christie J, Kozai T, Smith MAL (eds) Automation and environmental control in plant tissue culture. Springer, Dordrecht, pp 493–516
Ziv M, Schwartz A, Fleminger D (1987) Malfunctioning stomata in vitreous leaves of carnation (Sianthus caryopphyllus) plants propagated in vitro, implications for hardening. Plant Sci 52:127–134
Zobayed S (2006) Aeration in plant tissue culture. In: Dutta Gupta S, Ibaraki Y (eds) Plant tissue culture engineering. Springer, Dordrecht, pp 313–327
Zobayed SMA, Afreen F, Kozai T (2001) Physiology of Eucalyptus plantlets grown photoautotrophically in a scaled-up vessel. In Vitro Cell Dev Biol - Plant 37:807–813
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Editor: Baochun Li
Rights and permissions
About this article
Cite this article
Ferreira, L.S., Generoso, A.L., Carvalho, V.S. et al. Better light spectral quality and thermal amplitude inside the greenhouse stimulate growth and improve acclimatization of in vitro–grown Cattleya warneri T. Moore. In Vitro Cell.Dev.Biol.-Plant 57, 883–896 (2021). https://doi.org/10.1007/s11627-021-10162-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-021-10162-8