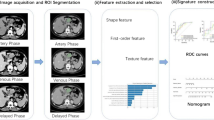
Abstract
Objective
The objective is to develop a mitotic prediction model and preoperative risk stratification nomogram for gastrointestinal stromal tumor (GIST) based on computed tomography (CT) radiomic features.
Methods
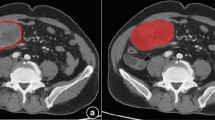
A total of 267 GIST patients from 2009.07 to 2015.09 were retrospectively collected and randomly divided into (6:4) training cohort and validation cohort. The 2D-tumor region of interest was delineated from the portal-phase images on contrast-enhanced (CE)-CT, and radiomic features were extracted. Lasso regression method was used to select valuable features to establish a radiomic model for predicting mitotic index in GIST. Finally, the nomogram of preoperative risk stratification was constructed by combining the radiomic features and clinical risk factors.
Results
Four radiomic features closely related to the level of mitosis were obtained, and a mitotic radiomic model was constructed. The area under the curve (AUC) of the radiomics signature model used to predict mitotic levels in training and validation cohorts (training cohort AUC = 0.752; 95% confidence interval [95%CI] 0.674–0.829; validation cohort AUC = 0.764; 95% CI 0.667–0.862). Finally, the preoperative risk stratification nomogram combining radiomic features was equivalent to the clinically recognized gold standard AUC (0.965 vs. 0.983) (p = 0.117). The Cox regression analysis found that the nomogram score was one of the independent risk factors for the long-term prognosis of the patients.
Conclusion
Preoperative CT radiomic features can effectively predict the level of mitosis in GIST, and combined with preoperative tumor size, accurate preoperative risk stratification can be performed to guide clinical decision-making and individualized treatment.




Similar content being viewed by others
Data availability
Data are available for bona fide researchers who request it from the authors.
Abbreviations
- GIST(s):
-
Gastrointestinal stromal tumor(s)
- CT:
-
Computed tomography
- ROI:
-
Region of interest
- CE-CT:
-
Contrast-enhanced computed tomography
- LASSO:
-
Least absolute shrinkage and selection operator
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- NIH:
-
National Institutes of Health
- NCCN:
-
National Comprehensive Cancer Network
- BMI:
-
Body mass index
- ICC:
-
Intraclass correlation coefficient
- GLCM:
-
Gray-level co-occurrence matrix
- GLRLM:
-
Gray-level run-length matrix
- GLSZM:
-
Gray-level size zone matrix
- GLDM:
-
Gray-level dependence matrix
- NGTDM:
-
Neighboring gray tone difference matrix
- ROC:
-
Receiver operating characteristic
- RFS:
-
Recurrence-free survival
- OS:
-
Overall survival
- HR:
-
Hazard ratio
References
Joensuu H, Hohenberger P, Corless CL (2013) Gastrointestinal stromal tumour. Lancet 382(9896):973–983. https://doi.org/10.1016/S0140-6736(13)60106-3
Parab TM, DeRogatis MJ, Boaz AM et al (2019) Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol 10:144–154. https://doi.org/10.21037/jgo.2018.08.20
Anderson WJ, Doyle LA (2021) Updates from the 2020 World Health Organization classification of soft tissue and bone tumours. Histopathology 78(5):644–657. https://doi.org/10.1111/his.14265
Mantese G (2019) Gastrointestinal stromal tumor: epidemiology, diagnosis, and treatment. Curr Opin Gastroenterol 35(6):555–559. https://doi.org/10.1097/MOG.0000000000000584
Akahoshi K, Oya M, Koga T, Shiratsuchi Y (2018) Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 24(26):2806–2817. https://doi.org/10.3748/wjg.v24.i26.2806
Minoda Y, Esaki M, Ihara E et al (2022) Auxiliary diagnosis of subepithelial lesions by impedance measurement during endoscopic ultrasound guided fine-needle biopsy. Gastrointest Endosc S0016–5107(22):02197–02206. https://doi.org/10.1016/j.gie.2022.11.022
Zhang Y, Renberg S, Papakonstantinou A et al (2022) Diagnosing gastrointestinal stromal tumors: the utility of fine-needle aspiration cytology versus biopsy. Cancer Med 14:2729–2734. https://doi.org/10.1002/cam4.4630
Christensen AW, Goldberg AF (2022) Mitotic count of fine needle aspiration material of gastrointestinal stromal tumours of the stomach underestimates actual mitotic count. Cytopathology 331:100–106. https://doi.org/10.1111/cyt.13050
Fernández J, Gómez-Ruiz AJ, Olivares V et al (2018) Clinical and pathological features of “small” GIST (≤2 cm): What is their prognostic value? Eur J Surg Oncol 44:580–586. https://doi.org/10.1016/j.ejso.2018.01.087
Hølmebakk T, Wiedswang AM, Meza-Zepeda LA et al (2021) Integrating anatomical, molecular and clinical risk factors in gastrointestinal stromal tumor of the stomach. Ann Surg Oncol 28(11):6837–6845. https://doi.org/10.1245/s10434-021-09605-8
Nishida T, Hølmebakk T, Raut CP, Rutkowski P (2019) Defining tumor rupture in gastrointestinal stromal tumor. Ann Surg Oncol 26(6):1669–1675. https://doi.org/10.1245/s10434-019-07297-9
Hølmebakk T, Hompland I, Bjerkehagen B et al (2018) Recurrence-free survival after resection of gastric gastrointestinal stromal tumors classified according to a strict definition of tumor rupture: a population-based study. Ann Surg Oncol 25(5):1133–1139. https://doi.org/10.1245/s10434-018-6353-5
Casali PG, Blay JY, Abecassis N et al (2022) Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 33(1):20–33. https://doi.org/10.1016/j.annonc.2021.09.005
van der Graaf WTA, Tielen R, Bonenkamp JJ et al (2018) Nationwide trends in the incidence and outcome of patients with gastrointestinal stromal tumour in the imatinib era. Br J Surg 105(8):1020–1027. https://doi.org/10.1002/bjs.10809
Randall RL, Benjamin RS, Boles S et al (2018) Soft Tissue Sarcoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16(5):536–563
Fairweather M, Balachandran VP, Li GZ et al (2018) Cytoreductive surgery for metastatic gastrointestinal stromal tumors treated with tyrosine kinase inhibitors: a 2-institutional analysis. Ann Surg 268(2):296–302. https://doi.org/10.1097/SLA.0000000000002281
Li J, Ye Y, Wang J et al (2017) Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res 29(4):281–293. https://doi.org/10.21147/j.issn.1000-9604.2017.04.01
Joensuu H (2008) Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39(10):1411–1419. https://doi.org/10.1016/j.humpath.2008.06.025
Li GZ, Raut CP (2019) Targeted therapy and personalized medicine in gastrointestinal stromal tumors: drug resistance, mechanisms, and treatment strategies. Onco Targets Ther 12:5123–5133. https://doi.org/10.2147/OTT.S180763
Yang J, Qingyao Wu, Lei Xu et al (2020) Integrating tumor and nodal radiomics to predict lymph node metastasis in gastric cancer. Radiother Oncol 150:89–96. https://doi.org/10.1016/j.radonc.2020.06.004
Wang Y, Liu W, Yang Yu et al (2020) CT radiomics nomogram for the preoperative prediction of lymph node metastasis in gastric cancer. Eur Radiol 30(2):976–986. https://doi.org/10.1007/s00330-019-06398-z
Li M, Zhang J, Dan Y et al (2020) A clinical-radiomics nomogram for the preoperative prediction of lymph node metastasis in colorectal cancer. J Transl Med 18(1):46. https://doi.org/10.1186/s12967-020-02215-0
Park S, Sham JG, Kawamoto S et al (2021) CT radiomics-based preoperative survival prediction in patients with pancreatic ductal adenocarcinoma. AJR Am J Roentgenol 217(5):1104–1112. https://doi.org/10.2214/AJR.20.23490
Jiang Y, Chen C, Xie J et al (2018) Radiomics signature of computed tomography imaging for prediction of survival and chemotherapeutic benefits in gastric cancer. EBioMedicine 36:171–182. https://doi.org/10.1016/j.ebiom.2018.09.007
Autorino R, Gui B, Panza G et al (2022) Radiomics-based prediction of two-year clinical outcome in locally advanced cervical cancer patients undergoing neoadjuvant chemoradiotherapy. Radiol Med 127(5):498–506. https://doi.org/10.1007/s11547-022-01482-9
Dongsheng Gu, Yabin Hu, Ding H et al (2019) CT radiomics may predict the grade of pancreatic neuroendocrine tumors: a multicenter study. Eur Radiol 29(12):6880–6890. https://doi.org/10.1007/s00330-019-06176-x
Sun R, Limkin EJ, Vakalopoulou M et al (2018) A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol 19(9):1180–1191. https://doi.org/10.1016/S1470-2045(18)30413-3
Zhou C, Duan X, Zhang X et al (2016) Predictive features of CT for risk stratifications in patients with primary gastrointestinal stromal tumour. Eur Radiol 26(9):3086–3093. https://doi.org/10.1007/s00330-015-4172-7
Zhang L, Kang L, Li G et al (2020) Computed tomography-based radiomics model for discriminating the risk stratification of gastrointestinal stromal tumors. Radiol Med 125(5):465–473. https://doi.org/10.1007/s11547-020-01138-6
Xue C, Yuan J, Lo GG et al (2021) Radiomics feature reliability assessed by intraclass correlation coefficient: a systematic review. Quant Imaging Med Surg 11(10):4431–4460. https://doi.org/10.21037/qims-21-86
Zwanenburg A, Vallières M, Abdalah MA et al (2020) The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 295(2):328–338. https://doi.org/10.1148/radiol.2020191145
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 77(21):e104–e107. https://doi.org/10.1158/0008-5472
Hepp T, Schmid M, Gefeller O, Waldmann E, Mayr A (2016) Approaches to regularized regression—a comparison between Gradient Boosting and the Lasso. Methods Inf Med 55(5):422–430. https://doi.org/10.3414/ME16-01-0033
Nattino G, Pennell ML, Lemeshow S (2020) Assessing the goodness of fit of logistic regression models in large samples: a modification of the Hosmer–Lemeshow test. Biometrics 76(2):549–560. https://doi.org/10.1111/biom.13249
Vassos N, Jakob J, Kähler G et al (2021) Preservation of organ function in locally advanced non-metastatic gastrointestinal stromal tumors (GIST) of the stomach by neoadjuvant imatinib therapy. Cancers (Basel) 13(4):586. https://doi.org/10.3390/cancers13040586
Marqueen KE, Moshier E, Bucksteinr M et al (2021) Neoadjuvant therapy for gastrointestinal stromal tumors: a propensity score-weighted analysis. Int J Cancer 149(1):177–185. https://doi.org/10.1002/ijc.33536
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762. https://doi.org/10.1038/nrclinonc.2017.141
Thawani R, McLane M, Beig N et al (2018) Radiomics and radiogenomics in lung cancer: a review for the clinician. Lung Cancer 115:34–41. https://doi.org/10.1016/j.lungcan.2017.10.015
Jiang Y, Wang W, Chen C et al (2019) Radiomics signature on computed tomography imaging: association with lymph node metastasis in patients with gastric cancer. Front Oncol 9:340. https://doi.org/10.3389/fonc.2019.00340
Zhao Y, Feng M, Wang M et al (2021) CT radiomics for the preoperative prediction of Ki67 index in gastrointestinal stromal tumors: a multi-center study. Front Oncol 11:689136. https://doi.org/10.3389/fonc.2021.689136
Wang F-H, Zheng H-L, Li J-T et al (2022) Prediction of recurrence-free survival and adjuvant therapy benefit in patients with gastrointestinal stromal tumors based on radiomics features. Radiol Med 127(10):1085–1097. https://doi.org/10.1007/s11547-022-01549-7
Palatresi D, Fedeli F, Danti G et al (2022) Correlation of CT radiomic features for GISTs with pathological classification and molecular subtypes: preliminary and monocentric experience. Radiol Med 127(2):117–128. https://doi.org/10.1007/s11547-021-01446-5
Wang C, Li H, Jiaerken Y et al (2019) Building CT radiomics-based models for preoperatively predicting malignant potential and mitotic count of gastrointestinal stromal tumors. Transl Oncol 12(9):1229–1236. https://doi.org/10.1016/j.tranon.2019.06.005
Kang B, Yuan X, Wang H et al (2021) Preoperative CT-based deep learning model for predicting risk stratification in patients with gastrointestinal stromal tumors. Front Oncol 11:750875. https://doi.org/10.3389/fonc.2021.750875
Wang Y, Wang Y, Ren J et al (2022) Malignancy risk of gastrointestinal stromal tumors evaluated with noninvasive radiomics: a multi-center study. Front Oncol 12:9643. https://doi.org/10.3389/fonc.2022.966743
Wang M, Feng Z, Zhou L et al (2021) Computed-tomography-based radiomics model for predicting the malignant potential of gastrointestinal stromal tumors preoperatively: a multi-classifier and multicenter study. Front Oncol 11:5847. https://doi.org/10.3389/fonc.2021.582847
Acknowledgements
We thank all patients, their families and all investigators involved in the present study. I would also like to thank my tutor, Professor Jian-Wei Xie.
Funding
This research was funded by Fujian Province medical “Innovation Double High” construction fund ([2021] No. 76).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by J-XL, F-HW and Z-KW. The first draft of the manuscript was written by J-XL, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Data statement
The authors declare that they had full access to all of the data in this study and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the institutional review board.
Informed consent
This study is a retrospective study, and patients' informed consent was waived with the approval of the institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, JX., Wang, FH., Wang, ZK. et al. Prediction of the mitotic index and preoperative risk stratification of gastrointestinal stromal tumors with CT radiomic features. Radiol med 128, 644–654 (2023). https://doi.org/10.1007/s11547-023-01637-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-023-01637-2