Abstract
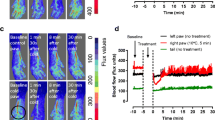
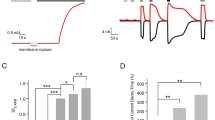
Sensing temperature is vitally important to adapt our body to environmental changes. Local warm detection is required to initiate regulation of cutaneous blood flow, which is part of the peripheral thermoregulatory mechanisms, and thus avoid damage to surrounding tissues. The mechanisms mediating cutaneous vasodilation during local heat stress are impaired with aging. However, the impact of aging on the ability of the skin to detect subtle thermal changes is unknown. Among heat-activated cation channels, transient receptor potential vanilloid 3 (TRPV3) is a thermo-sensor predominantly expressed on keratinocytes and involved in local vascular thermoregulatory mechanisms of the skin in young mice. In the present study, using a murine in vivo model of local heat exposure of the skin, we showed that heat-induced vasodilation was reduced in old mice associated with reduced expression of TRPV3 channels. We also found a decrease in expression and activity of TRPV3 channel, as well as reduced TRPV3-dependent adenosine tri-phosphate release in human primary keratinocytes from old donors. This study shows that aging alters the epidermal TRPV3 channels, which might delay the detection of changes in skin temperature, thereby limiting the mechanisms triggered for local vascular thermoregulation in the old skin.




Similar content being viewed by others
References
Kolarsick PAJ, Kolarsick MA, Goodwin C. Anatomy and physiology of the skin. J Dermatol Nurses Assoc. 2011;3:203. https://doi.org/10.1097/JDN.0b013e3182274a98.
Yousef H, Alhajj M, Sharma S. Anatomy, Skin (integument), epidermis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
Johnson JM, Minson CT, Kellogg DL. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol. 2014;4:33–89. https://doi.org/10.1002/cphy.c130015.
Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. https://doi.org/10.1152/physrev.00026.2005.
Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93:141–7. https://doi.org/10.1113/expphysiol.2007.038588.
Cracowski J-L, Roustit M. Human skin microcirculation. In: Comprehensive Physiology. John Wiley & Sons, Ltd; 2020. p. 1105–54.
Wong BJ, Hollowed CG. Current concepts of active vasodilation in human skin. Temperature (Austin). 2016;4:41–59. https://doi.org/10.1080/23328940.2016.1200203.
Smith CJ, Johnson JM. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: neural control of skin blood flow and sweating in humans. Auton Neurosci. 2016;196:25–36. https://doi.org/10.1016/j.autneu.2016.01.002.
Bentov I, Reed MJ. The effect of aging on the cutaneous microvasculature. Microvasc Res. 2015;100:25–31. https://doi.org/10.1016/j.mvr.2015.04.004.
El Assar M, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer CF, Rodríguez-Mañas L. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol. 2012;3:132. https://doi.org/10.3389/fphys.2012.00132.
Cau SBA, Carneiro FS, Tostes RC. Differential modulation of nitric oxide synthases in aging: therapeutic opportunities. Front Physiol. 2012;3:218. https://doi.org/10.3389/fphys.2012.00218.
Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci. 2010;15:718–39.
Balmain BN, Sabapathy S, Louis M, Morris NR. Aging and thermoregulatory control: the clinical implications of exercising under heat stress in older individuals. Biomed Res Int. 2018;2018 https://doi.org/10.1155/2018/8306154.
Greaney JL, Stanhewicz AE, Wolf ST, Kenney WL. Thermoregulatory reflex control of cutaneous vasodilation in healthy aging. Temperature (Austin). 8:176–87. https://doi.org/10.1080/23328940.2020.1832950.
Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 1985;2002(93):1644–9. https://doi.org/10.1152/japplphysiol.00229.2002.
Glatte P, Buchmann SJ, Hijazi MM, Illigens BM-W, Siepmann T. Architecture of the cutaneous autonomic nervous system. Front Neurol. 2019;0 https://doi.org/10.3389/fneur.2019.00970.
Stucky CL, Lewin GR. Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–505. https://doi.org/10.1523/JNEUROSCI.19-15-06497.1999.
Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. https://doi.org/10.1146/annurev.biochem.75.103004.142819.
Kashio M. Thermosensation involving thermo-TRPs. Mol Cell Endocrinol. 2021;520:111089. https://doi.org/10.1016/j.mce.2020.111089.
Voets T. TRP channels and thermosensation. In: Nilius B, Flockerzi V, editors. Mammalian transient receptor potential (TRP) cation channels: volume II, Handbook of Experimental Pharmacology. Cham: Springer International Publishing; 2014. p. 729–41.
Caterina MJ, Pang Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel). 2016;9 https://doi.org/10.3390/ph9040077.
Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z, et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010;12:130–41. https://doi.org/10.1016/j.cmet.2010.05.015.
Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci. 2011;31:1721–33. https://doi.org/10.1523/JNEUROSCI.4671-10.2011.
Peier AM. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–9. https://doi.org/10.1126/science.1073140.
Chung M-K, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–46. https://doi.org/10.1074/jbc.M303251200.
Fujii N, Kenny GP, McGarr GW, Amano T, Honda Y, Kondo N, Nishiyasu T. TRPV4 channel blockade does not modulate skin vasodilation and sweating during hyperthermia or cutaneous postocclusive reactive and thermal hyperemia. Am J Physiol Regul Integr Comp Physiol. 2021;320:R563–73. https://doi.org/10.1152/ajpregu.00123.2020.
Fromy B, Josset-Lamaugarny A, Aimond G, Pagnon-Minot A, Marics I, Tattersall GJ, Moqrich A, Sigaudo-Roussel D. Disruption of TRPV3 impairs heat-evoked vasodilation and thermoregulation: a critical role of CGRP. J Invest Dermatol. 2018;138:688–96. https://doi.org/10.1016/j.jid.2017.10.006.
Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–102. https://doi.org/10.1007/s00424-009-0703-x.
Huang SM, Lee H, Chung M-K, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28:13727–37. https://doi.org/10.1523/JNEUROSCI.5741-07.2008.
Seo SH, Kim S, Kim S-E, Chung S, Lee SE. Enhanced thermal sensitivity of TRPV3 in keratinocytes underlies heat-induced pruritogen release and pruritus in atopic dermatitis. J Invest Dermatol. 2020;140:2199–2209.e6. https://doi.org/10.1016/j.jid.2020.02.028.
Miyamoto T, Petrus MJ, Dubin AE, Patapoutian A. TRPV3 regulates NOS-independent nitric oxide synthesis in the skin. Nat Commun. 2011;2:369. https://doi.org/10.1038/ncomms1371.
Moqrich A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–72. https://doi.org/10.1126/science.1108609.
Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol. 1985;2010(109):1538–44. https://doi.org/10.1152/japplphysiol.00338.2010.
Fromy B, Sigaudo-Roussel D, Gaubert-Dahan M-L, Rousseau P, Abraham P, Benzoni D, Berrut G, Saumet JL. Aging-associated sensory neuropathy alters pressure-induced vasodilation in humans. J Invest Dermatol. 2010;130:849–55. https://doi.org/10.1038/jid.2009.279.
Raynard C, Ma X, Huna A, Tessier N, Massemin A, Zhu K, Flaman J-M, Moulin F, Goehrig D, Medard J-J, et al. NF-ΚB-dependent secretome of senescent cells can trigger neuroendocrine transdifferentiation of breast cancer cells. Aging Cell. 2022;21:e13632. https://doi.org/10.1111/acel.13632.
Szöllősi AG, Vasas N, Angyal Á, Kistamás K, Nánási PP, Mihály J, Béke G, Herczeg-Lisztes E, Szegedi A, Kawada N, et al. Activation of TRPV3 regulates inflammatory actions of human epidermal keratinocytes. J Invest Dermatol. 2018;138:365–74. https://doi.org/10.1016/j.jid.2017.07.852.
Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, Sinisi M, Birch R, Anand P. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. https://doi.org/10.1186/1471-2377-7-11.
Park CW, Kim HJ, Choi YW, Chung BY, Woo S-Y, Song D-K, Kim HO. TRPV3 channel in keratinocytes in scars with post-burn pruritus. Int J Mol Sci. 2017;18:2425. https://doi.org/10.3390/ijms18112425.
Muther C, Jobeili L, Garion M, Heraud S, Thepot A, Damour O, Lamartine J. An Expression screen for aged-dependent microRNAs identifies MiR-30a as a key regulator of aging features in human epidermis. Aging (Albany NY). 2017;9:2376–96. https://doi.org/10.18632/aging.101326.
Chevalier FP, Rorteau J, Ferraro S, Martin LS, Gonzalez-Torres A, Berthier A, El Kholti N, Lamartine J. MiR-30a-5p alters epidermal terminal differentiation during aging by regulating BNIP3L/NIX-dependent mitophagy. Cells. 2022;11:836. https://doi.org/10.3390/cells11050836.
Rorteau J, Chevalier FP, Bonnet S, Barthélemy T, Lopez-Gaydon A, Martin LS, Bechetoille N, Lamartine J. Maintenance of chronological aging features in culture of normal human dermal fibroblasts from old donors. Cells. 2022;11:858. https://doi.org/10.3390/cells11050858.
Broad LM, Mogg AJ, Eberle E, Tolley M, Li DL, Knopp KL. TRPV3 in drug development. Pharmaceuticals (Basel). 2016;9 https://doi.org/10.3390/ph9030055.
Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, Hatt H. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–40. https://doi.org/10.1038/sj.bjp.0707245.
Qi H, Shi Y, Wu H, Niu C, Sun X, Wang K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm Sin B. 2022;12:723–34. https://doi.org/10.1016/j.apsb.2021.08.002.
Chung M-K, Lee H, Mizuno A, Suzuki M, Caterina M. 2-Aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–82. https://doi.org/10.1523/JNEUROSCI.0934-04.2004.
Liu B, Yao J, Zhu MX, Qin F. Hysteresis of gating underlines sensitization of TRPV3 channels. J Gen Physiol. 2011;138:509–20. https://doi.org/10.1085/jgp.201110689.
Cheng X, Jin J, Hu L, Shen D, Dong X, Samie MA, Knoff J, Eisinger B, Liu M, Huang SM, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141:331–43. https://doi.org/10.1016/j.cell.2010.03.013.
Wang Y, Li H, Xue C, Chen H, Xue Y, Zhao F, Zhu MX, Cao Z. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol Toxicol. 2020; https://doi.org/10.1007/s10565-020-09536-2.
Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf). 2009;195:415–47. https://doi.org/10.1111/j.1748-1716.2009.01957.x.
Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95:269–80. https://doi.org/10.1093/cvr/cvs187.
Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic P2Y2 receptors mediate rapid Ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium. 2011;49:240–8. https://doi.org/10.1016/j.ceca.2011.02.008.
Burnstock G. Purine and purinergic receptors. Brain Neurosci Adv. 2018;2:2398212818817494. https://doi.org/10.1177/2398212818817494.
Gaubert ML, Sigaudo-Roussel D, Tartas M, Berrut G, Saumet JL, Fromy B. Endothelium-derived hyperpolarizing factor as an in vivo back-up mechanism in the cutaneous microcirculation in old mice. J Physiol. 2007;585:617–26. https://doi.org/10.1113/jphysiol.2007.143750.
Matz RL, de Sotomayor MA, Schott C, Stoclet J-C, Andriantsitohaina R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br J Pharmacol. 2000;131:303–11. https://doi.org/10.1038/sj.bjp.0703568.
DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–62. https://doi.org/10.1113/jphysiol.2002.019166.
Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284:H1662–7. https://doi.org/10.1152/ajpheart.00871.2002.
Škop V, Liu N, Guo J, Gavrilova O, Reitman ML. The contribution of the mouse tail to thermoregulation is modest. Am J Physiol Endocrinol Metab. 2020;319:E438–46. https://doi.org/10.1152/ajpendo.00133.2020.
Mota-Rojas D, Titto CG, de Mira Geraldo A, Martínez-Burnes J, Gómez J, Hernández-Ávalos I, Casas A, Domínguez A, José N, Bertoni A, et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals (Basel). 2021;11:3472. https://doi.org/10.3390/ani11123472.
Hankenson FC, Marx JO, Gordon CJ, David JM. Effects of rodent thermoregulation on animal models in the research environment. Comp Med. 2018;68:425–38. https://doi.org/10.30802/AALAS-CM-18-000049.
Lee YM, Kim YK, Chung JH. Increased expression of TRPV1 channel in intrinsically aged and photoaged human skin in vivo. Exp Dermatol. 2009;18:431–6. https://doi.org/10.1111/j.1600-0625.2008.00806.x.
Lee YM, Kang SM, Chung JH. The role of TRPV1 channel in aged human skin. J Dermatol Sci. 2012;65:81–5. https://doi.org/10.1016/j.jdermsci.2011.11.003.
Kashio M, Tominaga M. TRP channels in thermosensation. Curr Opin Neurobiol. 2022;75:102591. https://doi.org/10.1016/j.conb.2022.102591.
Johnson AJ, Wilson AT, Laffitte Nodarse C, Montesino-Goicolea S, Valdes-Hernandez PA, Somerville J, Peraza JA, Fillingim RB, Bialosky J, Cruz-Almeida Y. Age differences in multimodal quantitative sensory testing and associations with brain volume. Innov. Aging. 2021;5:igab033. https://doi.org/10.1093/geroni/igab033.
Heft MW, Robinson ME. Age differences in suprathreshold sensory function. Age (Dordr). 2014;36:1–8. https://doi.org/10.1007/s11357-013-9536-9.
Karttunen S, Duffield M, Scrimgeour NR, Squires L, Lim WL, Dallas ML, Scragg JL, Chicher J, Dave KA, Whitelaw ML, et al. Oxygen-dependent hydroxylation by FIH regulates the TRPV3 ion channel. J Cell Sci. 2015;128:225–31. https://doi.org/10.1242/jcs.158451.
Zhang N, Fu Z, Linke S, Chicher J, Gorman JJ, Visk D, Haddad GG, Poellinger L, Peet DJ, Powell F, et al. The asparaginyl hydroxylase factor inhibiting HIF-1α is an essential regulator of metabolism. Cell Metab. 2010;11:364–78. https://doi.org/10.1016/j.cmet.2010.03.001.
Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, Geurts AM, Palygin O, Stucky CL. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. Elife. 2018;7:e31684. https://doi.org/10.7554/eLife.31684.
Verkhratsky A, Burnstock G. Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. Bioessays. 2014;36:697–705. https://doi.org/10.1002/bies.201400024.
Fujii N, McGinn R, Halili L, Singh MS, Kondo N, Kenny GP. Cutaneous vascular and sweating responses to intradermal administration of ATP: a role for nitric oxide synthase and cyclooxygenase? J Physiol. 2015;593:2515–25. https://doi.org/10.1113/JP270147.
Fujii N, Halili L, Singh MS, Meade RD, Kenny GP. Intradermal administration of ATP augments methacholine-induced cutaneous vasodilation but not sweating in young males and females. Am J Physiol Regul Integr Comp Physiol. 2015;309:R912–9. https://doi.org/10.1152/ajpregu.00261.2015.
Talagas M, Lebonvallet N, Leschiera R, Marcorelles P, Misery L. What about physical contacts between epidermal keratinocytes and sensory neurons? Exp Dermatol. 2018;27:9–13. https://doi.org/10.1111/exd.13411.
Talagas M, Lebonvallet N, Leschiera R, Sinquin G, Elies P, Haftek M, Pennec J-P, Ressnikoff D, La Padula V, Le Garrec R, et al. Keratinocytes communicate with sensory neurons via synaptic-like contacts. Ann Neurol. 2020;88:1205–19. https://doi.org/10.1002/ana.25912.
Hou Q, Barr T, Gee L, Vickers J, Wymer J, Borsani E, Rodella L, Getsios S, Burdo T, Eisenberg E, et al. Keratinocyte expression of calcitonin gene-related peptide β: implications for neuropathic and inflammatory pain mechanisms. Pain. 2011;152:2036–51. https://doi.org/10.1016/j.pain.2011.04.033.
Thapa D, de Sousa Valente J, Barrett B, Smith MJ, Argunhan F, Lee SY, Nikitochkina S, Kodji X, Brain SD. Dysfunctional TRPM8 signalling in the vascular response to environmental cold in ageing. eLife. 2021;10:e70153. https://doi.org/10.7554/eLife.70153.
Acknowledgements
We acknowledge Jocelyne Vial and Geraldine Aimond for technical support and animal care facilities (AnexPeau facility, Lyon). We thank Theo Barthélemy and Emma Fraillon for their precious help for some experiments. We also thank Nicolas Lebonvallet, Ophelie Pierre, and Laurent Misery for their support and access to the calcium imaging platform of Laboratoire Interactions Epitheliums Neurones (LIEN/EA 4685/Brest). We thank Fabien Van Coppenolle (CarMeN/INSERM, U1060/Lyon) for his help in calcium imaging analysis and discussion. We thank PLATIM and especially Elodie Chatre for the microscopy. We thank Jerome Lamartine for critical reading of the manuscript.
Funding
This research project was supported by internal funds from CNRS and University Lyon 1. This work was supported by the French National Research Agency (KAST-ANR-23-CE14-0015).
Author information
Authors and Affiliations
Contributions
Conceptualization, L.S.M., F.P.C., and B.F.; methodology, L.S.M., A.J.-M., S.D., F.P.C., and B.F.; validation, L.S.M., T.E.J., F.P.C., and B.F.; formal analysis, L.S.M., A.J.-M., S.D., T.E.J., F.P.C., and B.F.; investigation, L.S.M., A.J.-M., S.D., F.P.C., and B.F.; resources, L.S.M., S.D., F.P.C., and B.F.; writing—original draft preparation, L.S.M., F.P.C., and B.F.; writing—review and editing, L.S.M., F.P.C., and B.F.; visualization, L.S.M., F.P.C., B.F.; S.D., T.E.J.; supervision, F.P.C. and B.F.; project administration, F.P.C. and B.F.; funding acquisition, F.P.C. and B.F.; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The animal study protocol was approved by the Animal Experimentation Committee of the University Claude Bernard Lyon I (protocol agreement #33946 approved on 25 November 2021).
Informed consent
Skin biopsies were obtained from the DermoBioTec tissue bank at Lyon (Tissue Transfer Agreement n_214854) with the informed consent of adult donors undergoing surgical discard (non-pathological tissues from breast, face, or abdomen), in accordance with the ethical guidelines (French Bioethics law of 2021).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 440 kb)
About this article
Cite this article
Martin, L.S., Josset-Lamaugarny, A., El Jammal, T. et al. Aging is associated with impaired triggering of TRPV3-mediated cutaneous vasodilation: a crucial process for local heat exposure. GeroScience (2023). https://doi.org/10.1007/s11357-023-00981-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-023-00981-5