Abstract
Introduction
Separately, both exercise and protein ingestion have been shown to alter the blood and urine metabolome. This study goes a step further and examines changes in the metabolome derived from blood, urine and muscle tissue extracts in response to resistance exercise combined with ingestion of three different protein sources.
Methods
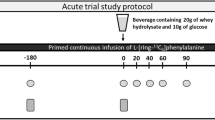
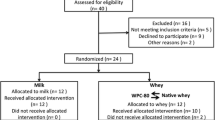
In an acute parallel study, 52 young males performed one-legged resistance exercise (leg extension, 4 × 10 repetitions at 10 repetition maximum) followed by ingestion of either cricket (insect), pea or whey protein (0.25 g protein/kg fat free mass). Blood and muscle tissue were collected at baseline and three hours after protein ingestion. Urine was collected at baseline and four hours after protein ingestion. Mixed-effects analyses were applied to examine the effect of the time (baseline vs. post), protein (cricket, pea, whey), and time x protein interaction.
Results
Nuclear magnetic resonance (NMR)-based metabolomics resulted in the annotation and quantification of 25 metabolites in blood, 35 in urine and 21 in muscle tissue. Changes in the muscle metabolome after combined exercise and protein intake indicated effects related to the protein source ingested. Muscle concentrations of leucine, methionine, glutamate and myo-inositol were higher after intake of whey protein compared to both cricket and pea protein. The blood metabolome revealed changes in a more ketogenic direction three hours after exercise reflecting that the trial was conducted after overnight fasting. Urinary concentration of trimethylamine N-oxide was significantly higher after ingestion of cricket than pea and whey protein.
Conclusion
The blood, urine and muscle metabolome showed different and supplementary responses to exercise and ingestion of the different protein sources, and in synergy the summarized results provided a more complete picture of the metabolic state of the body.




Similar content being viewed by others
Data Availability
Data reported in this paper will be made available upon request.
References
Berton, R., Conceição, M. S., Libardi, C. A., Canevarolo, R. R., Gáspari, A. F., Chacon-Mikahil, M. P. T., Zeri, A. C., & Cavaglieri, C. R. (2017). Metabolic time-course response after resistance exercise: A metabolomics approach. Journal of Sports Sciences, 35, 1211–1218.
Bertram, H. C. (2023). NMR foodomics in the assessment of diet and effects beyond nutrients. Current Opinion in Clinical Nutrition and Metabolic Care.
Bruno, C., Patin, F., Bocca, C., Nadal-Desbarats, L., Bonnier, F., Reynier, P., Emond, P., Vourc’h, P., Joseph-Delafont, K., Corcia, P., Andres, C. R., & Blasco, H. (2018). The combination of four analytical methods to explore skeletal muscle metabolomics: Better coverage of metabolic pathways or a marketing argument? Journal of Pharmaceutical and Biomedical Analysis, 148, 273–279.
Castro, A., Duft, R. G., Ferreira, M. L. V., Lugnani de Andrade, A. L., Gaspari, A. F., de Marchi Silva, L., 1, de Oliveira-Nunes, S. G., Cavaglieri, C. R., Ghosh, S., Bouchard, C., & Chacon- Mikahil, M. P. T. (2019). Association of skeletal muscle and serum metabolites with maximum power output gains in response to continuous endurance or high-intensity interval training programs: The TIMES study – A randomized controlled trialPLoS ONE 14(2): e0212115.
Costabile, G., Vetrani, C., Bozzetto, L., Giacco, R., Bresciani, L., Del Rio, D., Vitale, M., Della Pepa, G., Brighenti, F., Riccardi, G., Rivellese, A. A., & Annuzzi, G. (2021). Plasma TMAO increase after healthy diets: Results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. American Journal of Clinical Nutrition, 114, 1342–1350.
Cronin, O., Barton, W., Skuse, P., Penney, N. C., Garcia-Perez, I., Murphy, E. F., Woods, T., Nugent, H., Fanning, A., Melgar, S., Falvey, E. C., Holmes, E., Cotter, P. D., O’Sullivan, O., Molloy, M. G., & Shanahan, F. (2018). A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems 3.
Devries, M. C., & Phillips, S. M. (2015). Supplemental protein in support of muscle mass and health: Advantage whey. Journal of Food Science, 80(Suppl 1), A8–a15.
Dhakal, S., Moazzami, Z., Perry, C., & Dey, M. (2022). Effects of lean pork on Microbiota and Microbial-Metabolite Trimethylamine-N-Oxide: A Randomized Controlled Non-inferiority Feeding Trial based on the Dietary guidelines for americans. Molecular Nutrition & Food Research, 66, e2101136.
Enea, C., Seguin, F., Petitpas-Mulliez, J., Boildieu, N., Boisseau, N., Delpech, N., Diaz, V., Eugène, M., & Dugué, B. (2010). (1)H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Analytical and Bioanalytical Chemistry, 396, 1167–1176.
Evans, M., Cogan, K. E., & Egan, B. (2017). Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. Journal of Physiology, 595, 2857–2871.
Gibbons, H., O’Gorman, A., & Brennan, L. (2015). Metabolomics as a tool in nutritional research. Current Opinion in Lipidology, 26, 30–34.
Jang, H. J., Lee, J. D., Jeon, H. S., Kim, A. R., Kim, S., Lee, H. S., & Kim, K. B. (2018). Metabolic profiling of Eccentric Exercise-Induced muscle damage in human urine. Toxicol Res, 34, 199–210.
Jiménez, B., Holmes, E., Heude, C., Tolson, R. F., Harvey, N., Lodge, S. L., Chetwynd, A. J., Cannet, C., Fang, F., Pearce, J. T. M., Lewis, M. R., Viant, M. R., Lindon, J. C., Spraul, M., Schäfer, H., & Nicholson, J. K. (2018). Quantitative lipoprotein subclass and Low Molecular Weight Metabolite Analysis in Human serum and plasma by 1H NMR spectroscopy in a Multilaboratory Trial. Analytical Chemistry, 90, 11962–11971.
Khattri, R. B., Kim, K., Thome, T., Salyers, Z. R., O’Malley, K. A., Berceli, S. A., Scali, S. T., & Ryan, T. E. (2021). Unique Metabolomic Profile of Skeletal Muscle in chronic limb threatening ischemia. J Clin Med 10.
Khoramipour, K., Sandbakk, Ø., Keshteli, A. H., Gaeini, A. A., Wishart, D. S., & Chamari, K. (2022). Metabolomics in Exercise and sports: A systematic review. Sports Medicine (Auckland, N. Z.), 52, 547–583.
Kistner, S., Rist, M. J., Döring, M., Dörr, C., Neumann, R., Härtel, S., & Bub, A. (2020). An NMR-Based Approach to Identify Urinary Metabolites Associated with Acute Physical Exercise and Cardiorespiratory Fitness in Healthy Humans-Results of the KarMeN Study. Metabolites 10.
Krug, S., Kastenmüller, G., Stückler, F., Rist, M. J., Skurk, T., Sailer, M., Raffler, J., Römisch-Margl, W., Adamski, J., Prehn, C., Frank, T., Engel, K. H., Hofmann, T., Luy, B., Zimmermann, R., Moritz, F., Schmitt-Kopplin, P., Krumsiek, J., Kremer, W., Huber, F., Oeh, U., Theis, F. J., Szymczak, W., Hauner, H., Suhre, K., & Daniel, H. (2012). The dynamic range of the human metabolome revealed by challenges. Faseb j 26, 2607-19.
Lanng, S. K., Oxfeldt, M., Pedersen, S. S., Johansen, F. T., Risikesan, J., Lejel, T., Bertram, H. C., & Hansen, M. (2022). Influence of protein source (cricket, pea, whey) on amino acid bioavailability and activation of the mTORC1 signaling pathway after resistance exercise in healthy young males. European Journal of Nutrition.
Lima, A. R., Pinto, J., Barros-Silva, D., Jerónimo, C., Henrique, R., Bastos, M. L., Carvalho, M., & Pinho, G., P (2020). New findings on urinary Prostate cancer metabolome through combined GC-MS and (1)H NMR analytical platforms. Metabolomics, 16, 70.
Lombardo, M., Aulisa, G., Marcon, D., & Rizzo, G. (2022). The influence of animal- or plant-based diets on blood and urine Trimethylamine-N-Oxide (TMAO) levels in humans. Curr Nutr Rep, 11, 56–68.
Ma, H., Liu, X., Wu, Y., & Zhang, N. (2015). The Intervention Effects of Acupuncture on Fatigue Induced by Exhaustive Physical Exercises: A Metabolomics Investigation. Evid Based Complement Alternat Med 2015, 508302.
Madrid-Gambin, F., Llorach, R., Vázquez-Fresno, R., Urpi-Sarda, M., Almanza-Aguilera, E., Garcia-Aloy, M., Estruch, R., Corella, D., & Andres-Lacueva, C. (2017). Urinary (1)H nuclear magnetic resonance metabolomic fingerprinting reveals biomarkers of pulse consumption related to Energy-Metabolism Modulation in a Subcohort from the PREDIMED study. Journal of Proteome Research, 16, 1483–1491.
Madrid-Gambin, F., Brunius, C., Garcia-Aloy, M., Estruel-Amades, S., Landberg, R., & Andres-Lacueva, C. (2018). Untargeted (1)H NMR-Based metabolomics Analysis of urine and serum profiles after consumption of Lentils, Chickpeas, and beans: An extended meal study to Discover Dietary biomarkers of pulses. Journal of Agriculture and Food Chemistry, 66, 6997–7005.
Miccheli, A., Marini, F., Capuani, G., Miccheli, A. T., Delfini, M., Di Cocco, M. E., Puccetti, C., Paci, M., Rizzo, M., & Spataro, A. (2009). The influence of a sports drink on the postexercise metabolism of elite athletes as investigated by NMR-based metabolomics. Journal of the American College of Nutrition, 28, 553–564.
Morton, R., McGlory, C., & Phillips, S. (2015). Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Frontiers in Physiology 6.
Mukherjee, K., Edgett, B. A., Burrows, H. W., Castro, C., Griffin, J. L., Schwertani, A. G., Gurd, B. J., & Funk, C. D. (2014). Whole blood transcriptomics and urinary metabolomics to define adaptive biochemical pathways of high-intensity exercise in 50–60 year old masters athletes. PLoS One, 9, e92031.
Neal, C. M., Hunter, A. M., Brennan, L., O’Sullivan, A., Hamilton, D. L., De Vito, G., & Galloway, S. D. (2013). Six weeks of a polarized training-intensity distribution leads to greater physiological and performance adaptations than a threshold model in trained cyclists. J Appl Physiol (1985) 114, 461 – 71.
Paul, C., Laganà, A. S., Maniglio, P., Triolo, O., & Brady, D. M. (2016). Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecological Endocrinology, 32, 431–438.
Pechlivanis, A., Kostidis, S., Saraslanidis, P., Petridou, A., Tsalis, G., Mougios, V., Gika, H. G., Mikros, E., & Theodoridis, G. A. (2010a). (1)H NMR-based metabonomic investigation of the effect of two different exercise sessions on the metabolic fingerprint of human urine. Journal of Proteome Research, 9, 6405–6416.
Pechlivanis, A., Kostidis, S., Saraslanidis, P., Petridou, A., Tsalis, G., Mougios, V., Gika, H. G., Mikros, E., & Theodoridis, G. A. (2010b). 1H NMR-Based Metabonomic Investigation of the Effect of two different Exercise Sessions on the metabolic fingerprint of human urine. Journal of Proteome Research, 9, 6405–6416.
Pechlivanis, A., Papaioannou, K. G., Tsalis, G., Saraslanidis, P., Mougios, V., & Theodoridis, G. A. (2015). Monitoring the response of the human urinary metabolome to brief maximal Exercise by a combination of RP-UPLC-MS and (1)H NMR spectroscopy. Journal of Proteome Research, 14, 4610–4622.
Pellegrino, J. K., Anthony, T. G., Gillies, P., & Arent, S. M. (2022). The exercise metabolome: Acute aerobic and anaerobic signatures. Journal of the International Society of Sports Nutrition, 19, 603–622.
Saoi, M., Percival, M., Nemr, C., Li, A., Gibala, M., & Britz-McKibbin, P. (2019). Characterization of the Human Skeletal Muscle Metabolome for elucidating the mechanisms of bicarbonate ingestion on strenuous interval Exercise. Analytical Chemistry, 91, 4709–4718.
Sato, S., Parr, E. B., Devlin, B. L., Hawley, J. A., & Sassone-Corsi, P. (2018). Human metabolomics reveal daily variations under nutritional challenges specific to serum and skeletal muscle. Molecular Metabolism, 16, 1–11.
Sheedy, J. R., Gooley, P. R., Nahid, A., Tull, D. L., McConville, M. J., Kukuljan, S., Nowson, C. A., Daly, R. M., & Ebeling, P. R. (2014). (1)H-NMR analysis of the human urinary metabolome in response to an 18-month multi-component exercise program and calcium-vitamin-D3 supplementation in older men. Applied Physiology, Nutrition and Metabolism, 39, 1294–1304.
Skov, K., Oxfeldt, M., Thøgersen, R., Hansen, M., & Bertram, H. C. (2019). Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate-A Randomized Controlled Trial. Nutrients 11.
Tang, J. E., Moore, D. R., Kujbida, G. W., Tarnopolsky, M. A., & Phillips, S. M. (2009). Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985) 107, 987 – 92.
Thøgersen, R., Rasmussen, M. K., Sundekilde, U. K., Goethals, S. A., Van Hecke, T., Vossen, E., De Smet, S., & Bertram, H. C. (2020). Background Diet Influences TMAO Concentrations Associated with Red Meat Intake without Influencing Apparent Hepatic TMAO-Related Activity in a Porcine Model. Metabolites 10.
Thøgersen, R., Bertram, H. C., Vangsoe, M. T., & Hansen, M. (2021a). Krill Protein Hydrolysate Provides High Absorption Rate for All Essential Amino Acids-A Randomized Control Cross-Over Trial. Nutrients 13.
Thøgersen, R., Egsgaard, K. L., Kjølbæk, L., Jensen, K. J., Astrup, A., Hammershøj, M., Raben, A., & Bertram, H. C. (2021b). Effect of Dairy Matrix on the Postprandial Blood Metabolome. Nutrients 13.
Tipton, K. D., Ferrando, A. A., Phillips, S. M., Doyle, D. Jr., & Wolfe, R. R. (1999). Postexercise net protein synthesis in human muscle from orally administered amino acids. American Journal of Physiology, 276, E628–E634.
Wang, F., Han, J., He, Q., Geng, Z., Deng, Z., & Qiao, D. (2015). Applying 1H NMR Spectroscopy to Detect Changes in the Urinary Metabolite Levels of Chinese Half-Pipe Snowboarders after Different Exercises. Journal of Analytical Methods in Chemistry 2015, 315217.
Wang, Z., Bergeron, N., Levison, B. S., Li, X. S., Chiu, S., Jia, X., Koeth, R. A., Li, L., Wu, Y., Tang, W. H. W., Krauss, R. M., & Hazen, S. L. (2019). Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. European Heart Journal, 40, 583–594.
Yuhara, K., Yonehara, H., Hattori, T., Kobayashi, K., & Kirimura, K. (2015). Enzymatic characterization and gene identification of aconitate isomerase, an enzyme involved in assimilation of trans-aconitic acid, from Pseudomonas sp. WU-0701. The FEBS Journal, 282, 4257–4267.
Acknowledgements
This research was supported by grants from A.P. Møller Fonden, Denmark and Beckett Fonden, Denmark (#19-2-4811). The present study was part of Sofie Kaas Lanng’s Ph.D. project, which was funded by Centre for Innovative Food Research (CiFOOD), Aarhus University and HCB’s EliteForsk grant (#6161-00016B). NMR data were generated though accessing research infrastructure at Aarhus University, including FOODHAY (Food and Health Open Innovation Laboratory, Danish Roadmap for Research Infrastructure). The authors thank Janni Mosgaard Jensen and Gitte Kaiser Hartvigsen for technical support during the experimental days.
Author information
Authors and Affiliations
Contributions
SKL, MO, MH and HCB designed the experiment. SKL, MO, FTJ and TR conducted the experiments. SKL analysed the samples and performed the statistical analysis. SKL and HCB wrote the manuscript while all authors have read, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
This study was funded by the A.P. Møller Fonden, Copenhagen, Denmark and Beckett Foundation, Denmark (19-2-4811). The present study was part of Sofie Kaas Lanng’s PhD project, which was funded by Centre for Innovative Food Research (CiFOOD), Aarhus University and HCB’s Elite Research grant (6161-00016B). NMR data were generated though accessing research infrastructure at Aarhus University, including FOODHAY (Food and Health Open Innovation Laboratory, Danish Roadmap for Research Infrastructure).
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards the Declaration of Helsinki and the study was approved by The Central Denmark Region Committees on Health Research Ethics (journal nr. M-2019-291-19). Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lanng, S.K., Oxfeldt, M., Johansen, F.T. et al. Acute changes in the metabolome following resistance exercise combined with intake of different protein sources (cricket, pea, whey). Metabolomics 19, 98 (2023). https://doi.org/10.1007/s11306-023-02064-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-023-02064-0