Abstract
Background
Teprotumumab was approved by the US Food and Drug Administration (FDA) for the treatment of thyroid eye disease in 2020. However, its adverse events (AEs) have not been investigated in real-world settings.
Aim
This study aimed to detect and evaluate AEs associated with teprotumumab in the real-world setting by conducting a pharmacovigilance analysis of the FDA Adverse Event Reporting System (FAERS) database.
Method
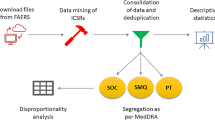
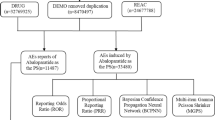
Reporting odds ratio (ROR) was used to detect risk signals from the data from January 2020 to March 2023 in the FAERS database.
Results
A total of 3,707,269 cases were retrieved, of which 1542 were related to teprotumumab. The FAERS analysis identified 99 teprotumumab-related AE signals in 14 System Organ Classes (SOCs). The most frequent AEs were muscle spasms (n = 287), fatigue (n = 174), blood glucose increase (n = 121), alopecia (n = 120), nausea (n = 118), hyperacusis (n = 117), and headache (n = 117). The AEs with strongest signal strengths were autophony (ROR = 14,475.49), deafness permanent (ROR = 1853.35), gingival recession (ROR = 190.74), deafness neurosensory (ROR = 129.89), nail growth abnormal (ROR = 103.67), onychoclasis (ROR = 73.58), ear discomfort (ROR = 72.88), and deafness bilateral (ROR = 62.46). Eleven positive AE signals were found at the standardized MedDRA queries (SMQs) level, of which the top five SMQs were hyperglycemia/new-onset diabetes mellitus, hearing impairment, gastrointestinal nonspecific symptoms and therapeutic procedures, noninfectious diarrhea, and hypertension. Age significantly increased the risk of hearing impairment.
Conclusion
This study identified potential new and unexpected AE signals of teprotumumab. Our findings emphasize the importance of pharmacovigilance analysis in the real world to identify and manage AEs effectively, ultimately improving patient safety during teprotumumab treatment.



Similar content being viewed by others
References
Ludgate M. Shining a light on thyroid eye disease. Nat Rev Endocrinol. 2020;16(5):259–60. https://doi.org/10.1038/s41574-020-0340-1.
Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43-g67. https://doi.org/10.1530/eje-21-0479.
Ross DS, Burch HB, Cooper DS, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–421. https://doi.org/10.1089/thy.2016.0229.
Slentz DH, Nelson CC, Smith TJ. Teprotumumab: a novel therapeutic monoclonal antibody for thyroid-associated ophthalmopathy. Expert Opin Investig Drugs. 2020;29(7):645–9. https://doi.org/10.1080/13543784.2020.1772752.
Ugradar S, Shi L, Wang Y, et al. Teprotumumab for non-inflammatory thyroid eye disease (TED): evidence for increased IGF-1R expression. Eye (Lond). 2021;35(9):2607–12. https://doi.org/10.1038/s41433-020-01297-w.
Kahaly GJ, Douglas RS, Holt RJ, et al. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diab Endocrinol. 2021;9(6):360–72. https://doi.org/10.1016/s2213-8587(21)00056-5.
Bartalena L, Marinò M, Marcocci C, et al. Teprotumumab for Graves’ orbitopathy and ototoxicity: moving problems from eyes to ears? J Endocrinol Invest. 2022;45(7):1455–7. https://doi.org/10.1007/s40618-022-01791-w.
Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–61. https://doi.org/10.1056/NEJMoa1614949.
Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341–52. https://doi.org/10.1056/NEJMoa1910434.
Chow A, Silkiss RZ. Teprotumumab-associated chronic hearing loss screening and proposed treatments. BMJ Case Rep. 2022;15:e248335. https://doi.org/10.1136/bcr-2021-248335.
Kessler DA. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. J Am Podiatr Med Assoc. 1994;84(1):35–8. https://doi.org/10.7547/87507315-84-1-35.
Wilson AM, Thabane L, Holbrook A. Application of data mining techniques in pharmacovigilance. Br J Clin Pharmacol. 2004;57(2):127–34. https://doi.org/10.1046/j.1365-2125.2003.01968.x.
Wan Q, Li Q, Lai X, et al. Data mining and safety analysis of BTK inhibitors: a pharmacovigilance investigation based on the FAERS database. Front Pharmacol. 2022;13: 995522. https://doi.org/10.3389/fphar.2022.995522.
Hu Y, Gong J, Zhang L, et al. Colitis following the use of immune checkpoint inhibitors: A real-world analysis of spontaneous reports submitted to the FDA adverse event reporting system. Int Immunopharmacol. 2020;84: 106601. https://doi.org/10.1016/j.intimp.2020.106601.
Böhm R, Höcker J, Cascorbi I, et al. OpenVigil–free eyeballs on FAERS pharmacovigilance data. Nat Biotechnol. 2012;30(2):137–8. https://doi.org/10.1038/nbt.2113.
Huang J, Jia Y, Sun S, et al. Adverse event profiles of dipeptidyl peptidase-4 inhibitors: data mining of the public version of the FDA adverse event reporting system. BMC Pharmacol Toxicol. 2020;21(1):68. https://doi.org/10.1186/s40360-020-00447-w.
Oshima Y, Tanimoto T, Yuji K, et al. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4(8):1112–5. https://doi.org/10.1001/jamaoncol.2017.4526.
van Puijenbroek EP, Bate A, Leufkens HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10. https://doi.org/10.1002/pds.668.
Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–36. https://doi.org/10.1002/pds.1742.
Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment: OPTIC-X study. Ophthalmology. 2022;129(4):438–49. https://doi.org/10.1016/j.ophtha.2021.10.017.
Shah K, Charitou M. A novel case of hyperglycemic hyperosmolar state after the use of teprotumumab in a patient with thyroid eye disease. AACE Clin Case Rep. 2022;8(4):148–9. https://doi.org/10.1016/j.aace.2022.01.004.
Safo MB, Silkiss RZ. A case of ulcerative colitis associated with teprotumumab treatment for thyroid eye disease. Am J Ophthalmol Case Rep. 2021;22: 101069. https://doi.org/10.1016/j.ajoc.2021.101069.
Ashraf DC, Jankovic I, El-Nachef N, et al. New-onset of inflammatory bowel disease in a patient treated with teprotumumab for thyroid associated ophthalmopathy. Ophthalmic Plast Reconstr Surg. 2021;37(5):e160–4. https://doi.org/10.1097/iop.0000000000001943.
Belinsky I, Creighton FX Jr, Mahoney N, et al. Teprotumumab and hearing loss: case series and proposal for audiologic monitoring. Ophthalmic Plast Reconstr Surg. 2022;38(1):73–8. https://doi.org/10.1097/iop.0000000000001995.
Lassale C, Batty GD, Steptoe A, et al. Insulin-like growth factor 1 in relation to future hearing impairment: findings from the english longitudinal study of ageing. Sci Rep. 2017;7(1):4212. https://doi.org/10.1038/s41598-017-04526-7.
Funding
This work was supported by the Shanghai Municipal Health Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Wang, Y., Qi, Z. et al. Data mining and analysis of adverse event signals associated with teprotumumab using the Food and Drug Administration adverse event reporting system database. Int J Clin Pharm 46, 471–479 (2024). https://doi.org/10.1007/s11096-023-01676-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01676-9