Abstract
Background
Antibody-drug conjugates have revolutionized cancer therapy due to their selectivity and efficacy. However, concerns have been raised regarding the potential effects of trastuzumab deruxtecan in interstitial lung diseases.
Aim
This study aimed to investigate the safety signals and time to onset of antibody-drug conjugates induced interstitial lung disease.
Method
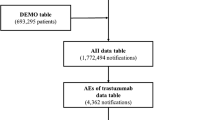
We utilized the FDA Adverse Event Reporting System database (2004–2022) to identify interstitial lung disease safety signals in 13 FDA-approved antibody-drug conjugates. Disproportionality analysis was conducted to estimate the reporting odds ratios for interstitial lung disease.
Results
Seven antibody-drug conjugates exhibited safety signals of interstitial lung disease: trastuzumab deruxtecan [reporting odds ratio, ROR (95% confidence intervals, CI) = 64.15 (57.07–72.10)], enfortumab vedotin [ROR (95% CI) = 5.24 (3.25–8.43)], trastuzumab emtansine [ROR (95% CI) = 3.62 (2.90–4.53)], brentuximab vedotin [ROR (95% CI) = 3.22 (2.49–4.17)], polatuzumab vedotin [ROR (95% CI) = 2.56 (1.59–4.12)], gemtuzumab ozogamicin [ROR (95% CI) = 2.53 (1.70–3.78)], and inotuzumab ozogamicin [ROR (95% CI) = 2.33 (1.21–4.49)]. Five antibody-drug conjugates with limited reports were excluded from further analysis: belantamab mafodotin, loncastuximab tesirine, mirvetuximab sorafenib, tisotumab vedotin, and moxetumomab pasudotox. Japan and the United States were the primary reporting countries.
Conclusion
This real-world study highlights high safety signals of interstitial lung disease associated with antibody-drug conjugates. Clinicians should be aware of these safety concerns and risk factors and implement early identification measures for their patients. Future research should prioritize comprehensively exploring the relationship between antibody-drug conjugates and lung diseases.




Similar content being viewed by others
Change history
17 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11096-024-01728-8
References
Tarantino P, Carmagnani PR, Corti C, et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. Ca-Cancer J Clin. 2022;72(2):165–82. https://doi.org/10.3322/caac.21705.
Wu Q, Qian W, Sun X, et al. Small-molecule inhibitors, immune checkpoint inhibitors, and more: fda-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J Hematol Oncol. 2022;15(1):143. https://doi.org/10.1186/s13045-022-01362-9.
Fu Z, Li S, Han S, et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Tar. 2022;7(1):93. https://doi.org/10.1038/s41392-022-00947-7.
Abuhelwa Z, Alloghbi A, Alqahtani A, et al. Trastuzumab deruxtecan-induced interstitial lung disease/pneumonitis in erbb2-positive advanced solid malignancies: a systematic review. Drugs. 2022;82(9):979–87. https://doi.org/10.1007/s40265-022-01736-w.
Hackshaw MD, Danysh HE, Singh J, et al. Incidence of pneumonitis/interstitial lung disease induced by her2-targeting therapy for her2-positive metastatic breast cancer. Breast Cancer Res Treat. 2020;183(1):23–39. https://doi.org/10.1007/s10549-020-05754-8.
Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated her2-positive breast cancer. New Engl J Med. 2020;382(7):610–21. https://doi.org/10.1056/NEJMoa1914510.
Tarantino P, Modi S, Tolaney SM, et al. Interstitial lung disease induced by anti-erbb2 antibody-drug conjugates: a review. JAMA Oncol. 2021;7(12):1873–81. https://doi.org/10.1001/jamaoncol.2021.3595.
Conte P, Ascierto PA, Patelli G, et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. Esmo Open. 2022;7(2): 100404. https://doi.org/10.1016/j.esmoop.2022.100404.
Zhu Z, Shen G, Li J, et al. Incidence of antibody-drug conjugates-related pneumonitis in patients with solid tumors: a systematic review and meta-analysis. Crit Rev Oncol Hemat. 2023;184: 103960. https://doi.org/10.1016/j.critrevonc.2023.103960.
Antoniou KM, Margaritopoulos GA, Tomassetti S, et al. Interstitial lung disease. Eur Respir Rev. 2014;23(131):40–54. https://doi.org/10.1183/09059180.00009113.
Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for her2-positive advanced breast cancer. New Engl J Med. 2012;367(19):1783–91. https://doi.org/10.1056/NEJMoa1209124.
Yoon S, Shin SJ, Kim HC, et al. Enfortumab vedotin-related pneumonitis is more common than expected and could lead to acute respiratory failure. Eur J Cancer. 2022;174:81–9. https://doi.org/10.1016/j.ejca.2022.07.014.
Ma Z, Zhang Y, Zhu M, et al. Interstitial lung disease associated with anti-her2 anti-body drug conjugates: results from clinical trials and the who’s pharmacovigilance database. Expert Rev Clin Phar. 2022;15(11):1351–61. https://doi.org/10.1080/17512433.2022.2121705.
Gastaldon C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the fda adverse event reporting system. Psychother Psychosom. 2021;90(1):41–8. https://doi.org/10.1159/000510703.
Hauben M, Bate A. Decision support methods for the detection of adverse events in post-marketing data. Drug Discov Today. 2009;14(7–8):343–57. https://doi.org/10.1016/j.drudis.2008.12.012.
FDA: Fda adverse event reporting system public dashboard. https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html (2023). Accessed 5 Mar 2023.
Capella D, Pedros C, Vidal X, et al. Case-population studies in pharmacoepidemiology. Drug Saf. 2002;25(1):7–19. https://doi.org/10.2165/00002018-200225010-00002.
Sakaeda T, Tamon A, Kadoyama K, et al. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10(7):796–803. https://doi.org/10.7150/ijms.6048.
Noguchi Y, Tachi T, Teramachi H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief Bioinform. 2021;22(6):bbab347. https://doi.org/10.1093/bib/bbab347.
Pace ND, Multani JK. On the reporting of odds ratios and risk ratios. Nutrients. 2018;10(10):1512. https://doi.org/10.3390/nu10101512.
van Puijenbroek EP, Bate A, Leufkens HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidem Drug Saf. 2002;11(1):3–10. https://doi.org/10.1002/pds.668.
Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet. 2022;400(10354):769–86. https://doi.org/10.1016/S0140-6736(22)01052-2.
Kubo K, Azuma A, Kanazawa M, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig. 2013;51(4):260–77. https://doi.org/10.1016/j.resinv.2013.09.001.
Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–68. https://doi.org/10.1200/JCO.2017.77.6385.
Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–42. https://doi.org/10.1093/annonc/mdx225.
Marinescu DC, Raghu G, Remy-Jardin M, et al. Integration and application of clinical practice guidelines for the diagnosis of idiopathic pulmonary fibrosis and fibrotic hypersensitivity pneumonitis. Chest. 2022;162(3):614–29. https://doi.org/10.1016/j.chest.2022.06.013.
Yong WP, Teo FS, Teo LL, et al. Clinical best practices in optimal monitoring, early diagnosis, and effective management of antibody-drug conjugate-induced interstitial lung disease or pneumonitis: a multidisciplinary team approach in Singapore. Expert Opin Drug Metab Toxicol. 2022;18(12):805–15. https://doi.org/10.1080/17425255.2022.2162383.
Izbicki G, Segel MJ, Christensen TG, et al. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002;83(3):111–9. https://doi.org/10.1046/j.1365-2613.2002.00220.x.
Read WL, Mortimer JE, Picus J. Severe interstitial pneumonitis associated with docetaxel administration. Cancer. 2002;94(3):847–53. https://doi.org/10.1002/cncr.10263.
Takeda T, Sasaki T, Fukuda K, et al. Risk factors for gemcitabine plus nab-paclitaxel-induced interstitial lung disease in pancreatic cancer patients. Int J Clin Oncol. 2021;26(3):543–51. https://doi.org/10.1007/s10147-020-01827-2.
Michielin O, Udry E, Periard D, et al. Irinotecan-induced interstitial pneumonia. Lancet Oncol. 2004;5(5):322–4. https://doi.org/10.1016/S1470-2045(04)01471-8.
Ohmori T, Yamaoka T, Ando K, et al. Molecular and clinical features of EGFR-TKI-associated lung injury. Int J Mol Sci. 2021;22(2):792. https://doi.org/10.3390/ijms22020792.
Oshima Y, Tanimoto T, Yuji K, et al. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4(8):1112–5. https://doi.org/10.1001/jamaoncol.2017.4526.
Zhang Y, Ma Z, Sun X, et al. Interstitial lung disease in patients treated with cyclin-dependent kinase 4/6 inhibitors: a systematic review and meta-analysis of randomized controlled trials. Breast. 2022;62:162–9. https://doi.org/10.1016/j.breast.2022.02.011.
Cortes J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. New Engl J Med. 2022;386(12):1143–54. https://doi.org/10.1056/NEJMoa2115022.
Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in japanese patients with lung cancer: a cohort and nested case-control study. Am J Resp Crit Care Med. 2008;177(12):1348–57. https://doi.org/10.1164/rccm.200710-1501OC.
Saito S, Lasky JA, Hagiwara K, et al. Ethnic differences in idiopathic pulmonary fibrosis: the Japanese perspective. Respir Investig. 2018;56(5):375–83. https://doi.org/10.1016/j.resinv.2018.06.002.
Imatoh T, Ushiki A, Ota M, et al. Association of hla-drb1*04:05 allele with drug-induced interstitial lung disease in Japanese population. Pharmacogenomics J. 2020;20(6):823–30. https://doi.org/10.1038/s41397-020-0172-3.
Wendisch D, Dietrich O, Mari T, et al. SARS-cov-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184(26):6243–61. https://doi.org/10.1016/j.cell.2021.11.033.
Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. New Engl J Med. 2021;16(384):1529–41. https://doi.org/10.1056/NEJMoa2028485.
Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase i/ii immu-132-01 basket trial. Ann Oncol. 2021;32(6):746–56. https://doi.org/10.1016/j.annonc.2021.03.005.
Kalinsky K, Diamond JR, Vahdat LT, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/her2-negative metastatic breast cancer: final results from a phase i/ii, single-arm, basket trial. Ann Oncol. 2020;31(12):1709–18. https://doi.org/10.1016/j.annonc.2020.09.004.
Rugo HS, Bardia A, Tolaney SM, et al. Tropics-02: a phase iii study investigating sacituzumab govitecan in the treatment of hr+/her2- metastatic breast cancer. Future Oncol. 2020;16(12):705–15. https://doi.org/10.2217/fon-2020-0163.
Tagawa ST, Balar AV, Petrylak DP, et al. Trophy-u-01: a phase ii open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39(22):2474–85. https://doi.org/10.1200/JCO.20.03489.
Kumagai K, Aida T, Tsuchiya Y, et al. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting ab-drug conjugate, in monkeys. Cancer Sci. 2020;111(12):4636–45. https://doi.org/10.1111/cas.14686.
Polakis P. Antibody drug conjugates for cancer therapy. Pharmacol Rev. 2016;68(1):3–19. https://doi.org/10.1124/pr.114.009373.
Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Resp Res. 2012;13(1):39. https://doi.org/10.1186/1465-9921-13-39.
Press MF, Lenz HJ. EGFR, HER2 and VEGF pathways: validated targets for cancer treatment. Drugs. 2007;67(14):2045–75. https://doi.org/10.2165/00003495-200767140-00006.
Spagnolo P, Bonniaud P, Rossi G, et al. Drug-induced interstitial lung disease. Eur Respir J. 2022;60(4):2102776. https://doi.org/10.1183/13993003.02776-2021.
Rugo HS, Tolaney SM, Loirat D, et al. Safety analyses from the phase 3 ascent trial of sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer. 2022;8(1):98. https://doi.org/10.1038/s41523-022-00467-1.
Seger D, Barker K, McNaughton C. Misuse of the Naranjo adverse drug reaction probability scale in toxicology. Clin Toxicol. 2013;51(6):461–6. https://doi.org/10.3109/15563650.2013.811588.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 82304629], and the Natural Science Foundation of Xiamen, China [Grant Numbers 3502Z202371048, 3502Z20227143].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The corresponding author Prof. Wei Zhuang’s e-mail address is corrected from 'zhuangw8@mail.sysu.edu.cn' to 'zhuangwei1333@gmail.com'.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, W., Xu, J., Liao, Y. et al. Assessing safety concerns of interstitial lung disease associated with antibody-drug conjugates: a real-world pharmacovigilance evaluation of the FDA adverse event reporting system. Int J Clin Pharm (2023). https://doi.org/10.1007/s11096-023-01673-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11096-023-01673-y