Abstract
Purpose
To evaluate the clinical feasibility and tolerability of large volume subcutaneous delivery at different injection depths for lean and non-lean subjects.
Methods
A single-center, randomized, subject-blinded, crossover study in 62 healthy subjects was conducted to evaluate delivery of a 10-cP solution containing hyaluronic acid. Subjects were separated into lean and non-lean cohort by SC thickness. A syringe pump was used to study the effect of different volumes (5, 12, 25 mL) of a viscous placebo solution and needle lengths (6, 9 and 12 mm) delivered at 0.5 mL/min.
Results
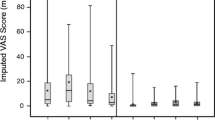
Across all treatments, injection sites were observed to have negligible leakage, ~34 kPa of back pressure, and VAS of mild pain with higher pain from needle insertion than during injection. While mild to moderate erythema was the most frequently reported ISR and edema was most prominent for 25 mL injections, all ISRs were resolved within 4 hours post injection. Subjects were unbothered by ISRs across all treatments and rated them as low distress scores (average 1.0–1.5 out of 6).
Conclusion
SC injection of 25 mL is feasible and tolerable using a low-pain formulation for abdomen injection irrespective of subcutaneous thickness and injection depths at a delivery rate of 0.5 mL/min.









Similar content being viewed by others
Abbreviations
- AE:
-
Adverse Events
- BD:
-
Beckton Dickinson
- cP:
-
Centipoise
- CRU:
-
Clinical Research Unity
- DAC:
-
Data Acquisition Module
- DP:
-
Drug Product
- GCP:
-
Good Clinical Practice
- ICH:
-
International Council for Harmonization
- ISP:
-
Injection Site Pain
- ISR:
-
Injection Site Reaction
- LVI:
-
Large Volume Injector
- Ph. Eur.:
-
European Pharmacopeia
- SC:
-
Subcutaneous
- SCIG:
-
Subcutaneous Immunoglobulin
- SE:
-
Standard Error
- USP:
-
United States Pharmacopeia
- VAS:
-
Visual Analog Scale
- WFI:
-
Water for Injection
References
Collins DS, Sanchez-Felix M, Badkar AV, Mrsny R. Accelerating the development of novel technologies and tools for the subcutaneous delivery of biotherapeutics. J Control Release. 2020;321:475–82.
Jones GB, Collins DS, Harrison MW, Thyagarajapuram NR, Wright JM. Subcutaneous drug delivery: An evolving enterprise. Sci Transl Med. 2017;9(405).
Badkar AV, Gandhi RB, Davis SP, LaBarre MJ. Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements. Drug Des Devel Ther. 2021;15:159–70.
Bittner B, Richter W, Schmidt J. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs. 2018;32(5):425–40.
Mathaes R, Koulov A, Joerg S, Mahler HC. Subcutaneous Injection Volume of Biopharmaceuticals-Pushing the Boundaries. J Pharm Sci. 2016;105(8):2255–9.
Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73(12):1307–19.
Shapiro R. Subcutaneous immunoglobulin therapy by rapid push is preferred to infusion by pump: a retrospective analysis. J Clin Immunol. 2010;30(2):301–7.
Klein JA, Jeske DR. Estimated Maximal Safe Dosages of Tumescent Lidocaine. Anesth Analg. 2016;122(5):1350–9.
Remington R, Hultman T. Hypodermoclysis to treat dehydration: a review of the evidence. J Am Geriatr Soc. 2007;55(12):2051–5.
Turner T, Cassano AM. Subcutaneous dextrose for rehydration of elderly patients–an evidence-based review. BMC Geriatr. 2004;4:2.
Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4(4):427–40.
Locke KW, Maneval DC, LaBarre MJ. ENHANZE((R)) drug delivery technology: a novel approach to subcutaneous administration using recombinant human hyaluronidase PH20. Drug Deliv. 2019;26(1):98–106.
Wasserman RL. Recombinant human hyaluronidase-facilitated subcutaneous immunoglobulin infusion in primary immunodeficiency diseases. Immunotherapy. 2017;9(12):1035–50.
Kang DW, Oh DA, Fu GY, Anderson JM, Zepeda ML. Porcine model to evaluate local tissue tolerability associated with subcutaneous delivery of protein. J Pharmacol Toxicol Methods. 2013;67(3):140–7.
Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: A phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53(2):192–201.
Hill SL, Davies A. Subcutaneous rituximab with recombinant human hyaluronidase in the treatment of non-Hodgkin lymphoma and chronic lymphocytic leukemia. Future Oncol. 2018;14(17):1691–9.
Shpilberg O, Jackisch C. Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase. Br J Cancer. 2013;109(6):1556–61.
Dias C, Abosaleem B, Crispino C, Gao B, Shaywitz A. Tolerability of High-Volume Subcutaneous Injections of a Viscous Placebo Buffer: A Randomized, Crossover Study in Healthy Subjects. AAPS PharmSciTech. 2015;16(5):1101–7.
Doughty DV, Clawson CZ, Lambert W, Subramony JA. Understanding Subcutaneous Tissue Pressure for Engineering Injection Devices for Large-Volume Protein Delivery. J Pharm Sci. 2016;105(7):2105–13.
Woodley WD, Morel DR, Sutter DE, Pettis RJ, Bolick NG. Clinical evaluation of large volume subcutaneous injection tissue effects, pain, and acceptability in healthy adults. Clin Transl Sci. 2022;15(1):92–104.
Allmendinger A. Opportunities in an Evolving Pharmaceutical Development Landscape: Product Differentiation of Biopharmaceutical Drug Products. Pharm Res. 2021;38(5):739–57.
Bittner B, Schmidt J. Subcutaneous drug delivery devices—Enablers of a flexible care setting. 2021. 159-79.
Prescribing Information: SKYRIZI® (risankizumab-rzaa) injection, for subcutaneous or intravenous use - Initial U.S. Approval: 2019 [Internet]. 2023 [cited 11 April 2023]. Available from: https://www.rxabbvie.com/pdf/skyrizi_pi.pdf.
Prescribing Information: REPATHA (evolocumab) injection, for subcutaneous use - Initial U.S. Approval: 2015 [Internet]. 2019 [cited 11 April 2023]. Available from: https://www.repatha.com/-/media/Themes/Amgen/Repatha-com/Repatha-com/PDFs/repatha_pi_hcp_english.pdf.
Beddoes C. Understanding the market for wearable large volume injectors. Health. 2016;44(1223): 928045.
Bedford T. Meeting emerging stakeholder needs with the subcuject wearable bolus injector. ONdrugDelivery Magazine; 2021. pp. 46–50.
Hooven MD, Joughin J. Wearable Large Volume Injectors Hold Promise for Success in Commercialization of Biologics. J Commercial Biotechnol. 2017;23(3):50–5.
Empaveli Injector (pegcetacoplan) - Instructions for Use [Internet]. 2023 [cited 12 October 2023]. Available from: https://pi.apellis.com/files/IFU_Empaveli_Injector.pdf.
Prescribing Information: Furoscix® (furosemide injection), for subcutaneous use - Initial U.S. Approval: 1968 [Internet]. 2022 [cited 11 April 2023]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/209988s000lbl.pdf.
Shi GH, Pisupati K, Parker JG, Corvari VJ, Payne CD, Xu W, et al. Subcutaneous Injection Site Pain of Formulation Matrices. Pharmaceutical Res. 2021;38(5):779–93.
Kaiser C, Knight A, Nordström D, Pettersson T, Fransson J, Florin-Robertsson E, et al. Injection-site reactions upon Kineret (anakinra) administration: experiences and explanations. Rheumatol Int. 2012;32(2):295–9.
Thomaidou E, Ramot Y. Injection site reactions with the use of biological agents. Dermatol Therap. 2019;32(2):e12817.
Zeltser R, Valle L, Tanck C, Holyst MM, Ritchlin C, Gaspari AA. Clinical, histological, and immunophenotypic characteristics of injection site reactions associated with etanercept: a recombinant tumor necrosis factor α receptor: Fc fusion protein. Archives Derma. 2001;137(7):893–9.
Rayner JE, Laino AM, Nufer KL, Adams L, Raphael AP, Menzies SW, et al. Clinical Perspective of 3D Total Body Photography for Early Detection and Screening of Melanoma. Front Med (Lausanne). 2018;5:152.
Skvara H, Burnett P, Jones J, Duschek N, Plassmann P, Thirion JP. Quantification of skin lesions with a 3D stereovision camera system: validation and clinical applications. Skin Res Technol. 2013;19(1):e182-90.
Ignaut DA, Fu H. Comparison of Insulin Diluent Leakage Postinjection Using Two Different Needle Lengths and Injection Volumes in Obese Patients with Type 1 or Type 2 Diabetes Mellitus. J Diabetes Sci Technol. 2012;6(2):389–93.
Huskisson EC. Measurement of pain. J Rheumatol. 1982;9(5):768–9.
Langley GB, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int. 1985;5(4):145–8.
Montenegro ML, Braz CA, Mateus-Vasconcelos EL, Rosa-e-Silva JC, Candido-dos-Reis FJ, Nogueira AA, et al. Pain pressure threshold algometry of the abdominal wall in healthy women. Braz J Med Biol Res. 2012;45(7):578–82.
Præstmark KA, Stallknecht B, Jensen ML, Sparre T, Madsen NB, Kildegaard J. Injection Technique and Pen Needle Design Affect Leakage From Skin After Subcutaneous Injections. J Diabetes Sci Technol. 2016;10(4):914–22.
Berteau C, Filipe-Santos O, Wang T, Rojas HE, Granger C, Schwarzenbach F. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med Devices (Auckl). 2015;8:473–84.
Nash P, Vanhoof J, Hall S, Arulmani U, Tarzynski-Potempa R, Unnebrink K, et al. Randomized Crossover Comparison of Injection Site Pain with 40 mg/0.4 or 0.8 mL Formulations of Adalimumab in Patients with Rheumatoid Arthritis. Rheumatology and Therapy. 2016;3(2):257-70.
Bodian Carol A, Freedman G, Hossain S, Eisenkraft James B, Beilin Y. The Visual Analog Scale for Pain: Clinical Significance in Postoperative Patients. Anesthesiology. 2001;95(6):1356–61.
Pager A. 8mm needle – improving subcutaneous chronic drug delivery. ONdrugDelivery Magazine; 2019. pp. 28–32.
Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3(1):41–9.
Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23(1–2):37–43.
Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Current Medical Research and Opinion. 2010;26(6):1519–30.
Acknowledgements
The authors would like to thank the volunteers and all investigators involved in this clinical trial study. Andrew M Ratz, Kaoutar Abbou Oucherif, Amita Datta-Mannan, Michelle Yi Xiu Lim, and Rhea Sirkar contributed to the discussions, design, and execution of the studies. Reshma Bharadwaj contributed to the review of the manuscript.
Funding
Funding for the non-clinical and clinical studies was provided by Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Listed authors are employees of and hold shares in Eli Lilly and Company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dang, X., Shih, H., Sharma, R. et al. Clinical Investigation of Large Volume Subcutaneous Delivery up to 25 mL for Lean and Non-Lean Subjects. Pharm Res 41, 751–763 (2024). https://doi.org/10.1007/s11095-024-03683-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-024-03683-5