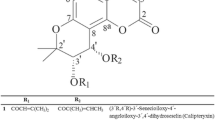
Biological activity of the Biginelli type heterocycles is extremely broad and provides a suitable platform for the discovery of potent small drug-like molecules. Such activity of 3,4-dihydropyrimidin-2(1H)-one (DHPM) derivatives is widely known, whereas their oxygen-bridged analogs, benzoxadiazocines, are presented quite rarely in the literature. In this study, a series of new methano[1,2,4]triazolo[1,5-c][1,3,5]benzoxadiazocine derivatives (3a-3j) were evaluated in vitro for their activities and molecular docking features. According to the molecular docking study, COX-2 and PGE2S appeared as likely targets responsible for the reduced PGE2 levels caused by the title compounds. The cytotoxicity of compounds 3a-3g, 3j was evaluated on RAW 264.7 murine macrophage cell line by MTT assay after treatment for 24 h with various doses (25, 50, 100 μM) of these compounds. Then, compounds admitting cell viability higher than 70% were tested for their anti-inflammatory activity at non-toxic doses by evaluating the nitrite level of cell supernatants with the Griess reagent. Compounds 3c and 3f demonstrated significant inhibition of nitrite production (by 29 and 25%, respectively) at 100 μM (p < 0.05). These compounds significantly inhibited PGE2 production, thus suggesting analgesic activity.







Similar content being viewed by others
References
G. C. Tron, A. Minassi, and G. Appendino, Eur. J. Org. Chem., 28, 5541 – 5550 (2011). https: //doi.org/https://doi.org/10.1002/ejoc.201100661
S. Sandhu and J. S. Sandhu, Arkivoc (i) 66 – 133 (2012).
J. P. Wan and Y. Pan, Mini-Rev. Med. Chem. 12, 337 – 349 (2012). https: //doi.org/https://doi.org/10.2174/138955712799829267
A. de Fatima, T. C. Braga, L. S. da Neto, et al., J. Adv. Res. 6(3), 363 – 373 (2015). https: doi.org/https://doi.org/10.1016/j.jare.2014.10.006
H. Nagarajaiah, A. Mukhopadhyay, and J. N. Moorthy, Tetrahedron Lett. 57, 5135 – 5149 (2016). https: //doi.org/https://doi.org/10.1016/j.tetlet.2016.09.047
O. P. Shkurko, T. G. Tolstikova, and V. F. Sedova, Russ. Chem. Rev., 85, 1056 – 1096 (2016). https: //doi.org/https://doi.org/10.1070/RCR4586
E. M. H. Abbas, S. M. Abdallah, M. H. Abdoh, et al., Turk. J. Chem. 32, 297 – 304 (2008).
L. H. S. Matos, F. T. Masson, L. A. Simeoni, et al., Eur. J. Med. Chem., 143 1779 – 1789 (2018). https: //doi.org/https://doi.org/10.1016/j.ejmech.2017.10.073
J. J. Baldwin, D. A. Claremon, and D. E. McClure, US Patent 4609494A (1986).
İ. S. Zorkun, S. Saraç, S. Çelebi, et al., Bioorg. Med. Chem., 14, 8582 – 8589 (2006). https: //doi.org/https://doi.org/10.1016/j.bmc.2006.08.031
B. L. Finkelstein, E. A. Benner, and M. C. Hendrixson, et al., Bioorg. Med. Chem., 10, 599 – 613 (2002). https: //doi.org/https://doi.org/10.1016/S0968-0896(01)00326-1
W. S. El-Hamouly, A-M. A. El-Khamry, and E. M. H. Abbas, Indian J. Chem., 45B, 2091 – 2098 (2006).
M. Yar, M. Bajda, L. Shahzadi, et al., Bioorg. Chem., 54, 96 – 104 (2014). https: //doi.org/https://doi.org/10.1016/j.bioorg.2014.05.003
G. Karageorgis, E. S. Reckzeh, and J. Ceballos, et al., Nature Chem., 10, 1103 – 1111 (2018). https: //doi.org/https://doi.org/10.1038/s41557-018-0132-6
N. Jankoviæ, T. J. Ristovski, M. Vraneš, et al. Bioorg. Chem., 86, 569 – 582 (2019). https: //doi.org/https://doi.org/10.1016/j.bioorg.2019.02.026
N. Y. Gorobets, Y. V. Sedash, K. S. Ostras, et al. Tetrahedron Lett., 51, 2095 – 2098 (2010). https: //doi.org/https://doi.org/10.1016/j.tetlet.2010.02.045
Y. V. Sedash, N. Y. Gorobets, V. A. Chebanov, et al., RSC Adv., 2, 6719 – 6728 (2012). https: //doi.org/https://doi.org/10.1039/C2RA20195J
V. A. Peshkov, A. A. Peshkov, O. P. Pereshivko, et al. ACS Comb. Sci., 16, 535 – 542 (2014). https: //doi.org/https://doi.org/10.1021/co5000695
M. Kondratiuk, N. Y. Gorobets, Y. V. Sedash, et al., Molbank, 2, M898 (2016). https: //doi.org/https://doi.org/10.3390/M898
M. K. Gümüş, N. Y. Gorobets, Y. V. Sedash, et al., Tetrahedron Lett., 58, 3446 – 3448 (2017). https: //doi.org/https://doi.org/10.1016/j.tetlet.2017.07.071
S. A. Komykhov, A. A. Bondarenko, V. I. Musatov, et al., Chem. Heterocycl. Comp., 53, 378 – 380 (2017). https: //doi.org/https://doi.org/10.1007/s10593-017-2059-z
M. V. Murlykina, A. D. Morozova, I. M. Zviagin, et al., Front. Chem., 6, 527 (2018) https: //doi.org/https://doi.org/10.3389/fchem.2018.00527
Y. I. Sakhno, M. V. Murlykina, O. I. Zbruyev, et al., Beilstein J. Org. Chem., 16, 281 – 289 (2020). https: //doi.org/https://doi.org/10.3762/bjoc.16.27
M. V. Murlykina, Y. I. Sakhno, S. M. Desenko, et al., Eur. J. Org. Chem., 20, 4481 – 4492 (2015). https: //doi.org/https://doi.org/10.1002/ejoc.201500469
M. K. Gümüş, N. Y. Gorobets, Y. V. Sedash, et al., Chem. Heterocyc. Comp., 53, 1261 – 1267 (2017). https: //doi.org/https://doi.org/10.1007/s10593-018-2204-3
M. K. Gümüş, S. Kansýz, E. Aydemir, et al., J. Mol. Struct., 1168, 280 – 290 (2018). https: //doi.org/https://doi.org/10.1016/j.molstruc.2018.05.032
M. K. Gümüş, S. Kansýz, C. Y. Ataol, et al., Acta Cryst. E, 75, 492 – 498 (2019). https: //doi.org/https://doi.org/10.1107/S2056989019003700
M. K. Gümüş, S. Kansýz, N. Dege, et al., Acta Cryst. E, 74, 1211 – 1214 (2018). https: //doi.org/https://doi.org/10.1107/S2056989018010848
E. Aydemir, S. Kansýz, M. K. Gümüş, et al., Acta Cryst. E, 74, 367 – 370 (2018). https: //doi.org/https://doi.org/10.1107/S2056989018002621
E. Tornqvist, A. Annas, B. Granath, et al., PLoS One, 9(7), e101638 (2014). doi:https://doi.org/10.1371/journal.pone.0101638
M. Koksal, A. Dedeoglu-Erdogan, M. Bader, et al., Arch. Pharm., e2000469 (2021). https: //doi.org/https://doi.org/10.1002/ardp.202000469
I. Erol, N. Okur, D. Orak, et al., Pharm. Dev. Tech., 25(8), 909 – 918 (2020). https: //doi.org/https://doi.org/10.1080/10837450.2020.1765180
K. Buran, R. Reis, H. Sipahi, et al., Arch. Pharm., e2000354 (2021). https: //doi.org/https://doi.org/10.1002/ardp.202000354
A. K. Kiemer and A. M. Vollmar, Endocrinology, 138, 4282 – 4290 (1997). https: //doi.org/https://doi.org/10.1210/endo.138.10.5459
P. M. Giang, N. T. Nga, B. Van Trung, et al., Pharm. Chem. J. 53, 628 – 634 (2019). https: //doi.org/https://doi.org/10.1007/s11094-019-02051-7
C. R. Lin, F. Amaya, L. Barrett, et al., J. Pharm. Exp. Ther., 319, 1096 – 1103 (2006). https: //doi.org/https://doi.org/10.1124/jpet.106.105569
E. Harder,W. Damm, J. Maple, et al., J. Chem. Theory Comput., 12, 281 – 296 (2016). https: //doi.org/https://doi.org/10.1021/acs.jctc.5b00864
T. Yamada, J. Komoto, K. Watanabe, et al., J Mol Biol., 348, 1163 – 1176 (2005). https: //doi.org/https://doi.org/10.1016/j.jmb.2005.03.035
K. Gupta, B. S. Selinsky, and P. J. Loll, Acta Cryst. D, 62, 151 – 156 (2006). https: //doi.org/https://doi.org/10.1107/S0907444905036309
B. J. Orlando and M. G. Malkowski, J. Biol. Chem., 291, 15069 – 15081 (2016). https: //doi.org/https://doi.org/10.1074/jbc.M116.725713
H. M. Berman, J.Westbrook, Z. Feng, et al., Nucleic Acids Res., 28, 235 – 242 (2000). https: //doi.org/https://doi.org/10.1093/nar/28.1.235
G. M. Sastry, M. Adzhigirey, T. Day, et al., J. Comput. Aided Mol. Des., 27, 221 – 234 (2013). https: //doi.org/https://doi.org/10.1007/s10822-013-9644-8
R. A. Friesner, R. B. Murphy, M. P. Repasky, et al., J. Med. Chem., 49, 6177 – 6196 (2006). https: //doi.org/https://doi.org/10.1021/jm051256o
C. A. Lipinski, F. Lombardo, B. W. Dominy, et al., Adv. Drug Deliv. Rev., 46, 3 – 26 (2001). https: //doi.org/https://doi.org/10.1016/S0169-409X(96)00423-1
D. F. Veber, S. R. Johnson, H. Y. Cheng, et al., J. Med. Chem., 45, 2615 – 2623 (2002). https: //doi.org/https://doi.org/10.1021/jm020017n
J. Baell and M. A. Walters. Nature, 513(7519), 481 – 483 (2014).
S. Sari, Hacettepe Uni. J. Fac. Pharm., 40, 34 – 47 (2020). https: //dergipark.org.tr/en/pub/hujpharm/issue/54931/722540
Z. Fang, Y. N. Song, P. Zhan, et al., Future Med. Chem., 6, 885 – 901 (2014). https: //doi.org/https://doi.org/10.4155/fmc.14.50
D. L. Simmons, R. M. Botting, and T. Hla, Pharm. Rev., 56, 387 – 437 (2004). https: //doi.org/https://doi.org/10.1124/pr.56.3.3
M. Murakami and I. Kudo, Curr. Pharm. Des., 12, 943 – 954 (2006). https: //doi.org/https://doi.org/10.2174/138161206776055912
J. P. Iyer, P. K. Srivastava, R. Dev, et al., Expert Opinion Therapeutic Targets, 13, 849 – 865 (2009). https: //doi.org/https://doi.org/10.1517/14728220903018932
S. W. Rowlinson, J. R. Kiefer, J. J. Prusakiewicz, et al., J. Biol. Chem., 278, 45763 – 45769 (2003). https: //doi.org/https://doi.org/10.1074/jbc.M305481200
B. J. Orlando and M. G. Malkowski, Acta Cryst. F, 72, 772 – 776 (2016). https: //doi.org/https://doi.org/10.1107/S2053230X16014230
M. J. Lucido, B. J. Orlando, A. J. Vecchio, et al., Biochemistry, 55, 1226 – 1238 (2016). https: //doi.org/https://doi.org/10.1021/acs.biochem.5b01378
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doğan, İ.S., Gümüş, M.K., Gorobets, N.Y. et al. In Vitro Cytotoxicity of Methano[1,2,4]Triazolo-[1,5-C][1,3,5]Benzoxadiazocine Derivatives and Their Effects on Nitrite and Prostaglandin E2 (PGE2) Levels. Pharm Chem J 56, 769–776 (2022). https://doi.org/10.1007/s11094-022-02708-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02708-w