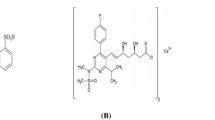
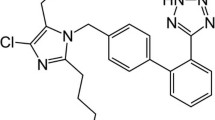
A reverse-phase high performance liquid chromatography (RP-HPLC) method was developed and validated for simultaneous estimation of fimasartan (FIM), amlodipine besylate (AML) and rosuvastatin calcium (ROS) in combination for the treatment of hypertension with dyslipidemia presenting a major risk factor for cardiovascular diseases. Chromatographic separation was performed on stationary reverse-phase inert sustain-swift C18 100A° (250 mm × 4.6 mm, 5 μm i.d.) column. The optimized mobile phase acetonitrile – methanol – water (15:15:70, v/v, at pH 3.5 adjusted with 0.1% ortho-phosphoric acid) was pumped at a flow rate of 1.0 mL/min and the effluent spectrum was monitored at 230 nm. Calibration graphs of drugs constructed at their wavelengths of determination were linear in a concentration range of 10 – 100 μg/mL for all three components. The retention times for FIM, ROS calcium, and AML peaks at 230 nm were found to be 4.6, 6.4, and 10.8 min respectively. The method suggested usefulness of a unique mobile phase during the estimation of two or more multicomponent dosage forms. Due to its simplicity, rapidness, high precision, and accuracy, the proposed RP-HPLC method can be applied for simultaneous determination of FIM, ROS and AML in bulk mixture.









Similar content being viewed by others
References
S. Kumar and B. Ram., J. Drug Delivery Ther., 9(1), 463 – 466 (2019).
A. Pradhan, V. Gupta, and R. Sethi., J. Family. Med. Prim. Care., 8, 2184 – 8 (2019).
P. S. Jain, M. K. Patel, S. B. Bari., et al., Indian J. Pharm. Sci., 74(2), 152–156 (2012).
T. Hemant, S. D. Swathi, R. K. Vara, et al., Int. J. Pharm. Sci. Res., 6(7), 2913 – 17, (2015).
H. M. Akiful, P. K. Shanthi, and M. Dibyalochan, Asian J. Pharm. Clin. Res., 11(7), 393 – 397 (2018).
D. A. Sica., Drugs, 62(3), 443 – 62 (2002).
J. Prajapati, A. Patel, and M. B. Patel., Int. J. PharmTech Res., 3(2), 801 – 808 (2011).
P. Sahu and V. B. Patel., Indian J. Pharm. Sci.., 69(1), 110 – 111 (2007).
K. P. Patel, U. K. Chhalotiya, H. M. Kachhiya, and J. M. Patel, Future J. Pharm. Sci., 6(1), 1 – 9 (2020).
R. N. Shah, D. B. Gandhi, and M. M. Patel., Int. J. Pharm. Pharm. Sci., 5, 633 – 636 (2012).
S. Yi, J. Kim, and T. Kim., J. Clin. Pharmacol., 49, 321 – 327 (2011).
O. Minkyung, G. Jong-Lyul, P. Sung-Eun, et al., Drug Des. Dev. Ther., 12, 1157 – 1164 (2018).
H. Jeon, K. S. Lim, and H. I. Shin., J. Cardiovasc. Pharmacol., 59, 84 – 91 (2012).
M. Gumustas., J. AOAC Int., 96, 751 – 757 (2013).
Lee han-soo, Combination Drug Rave, Boryung’s Combination Drug Kanarb Targets Global Market, Korea BioMed Review (2020).
B. Dhandapani, N. Anjaneyulu, and Y. Venkateshwarlu., J. Pharm. Res., 3(2), 332 – 334 (2010)
K. Devansh, K. Usmangani, and H. M. Chhalotiya., J. Chem. Metrol., 14(2), 142 – 152 (2020).
Patent No. NCT03156842, U. S. National Library of Medicine (2019), Combination of Fimsartan / Amlodipine / Rosuvastatin in Patients with Essential Hypertension and Dyslipidaemia by Boryung Pharmaceutical. https://clinicaltrials.gov/ct2/show/NCT03156842.(2019).
Eur. Patent No. EP2974719A1, Pharmaceutical Combination Drugs by Boryung Pharmaceutical (2019).
International Conference on Harmonization (ICH). Technical Requirements for Registration of Pharmaceuticals for Human Use: Stability Testing of New Drug Substancex and Products, ICH Q2 (R1) (2003).
S. Chandran and R. S. Singh., Pharmazie, 62, 4 – 14 (2007).
Quality Assurance of Pharmaceuticals, Compendium of Guidelines and Related Materials, WHO, Geneva (1997), Vol. I, pp. 119 – 124.
Acknowledgements
The authors are thankful to Glenmark Pharmaceuticals Ltd., Alkem Laboratories, and Chandra Labs Ltd. (India) for providing reference standards and to Laxminarayan Dev College of Pharmacy for providing all facilities to complete this research work.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors have equally contributed to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mistry, R.P., Shah, C. & Jat, R. A New Reverse-Phase High Performance Liquid Chromatography Method for Simultaneous Estimation of Fimasartan, Rosuvastatin Calcium, and Amlodipine Besylate in Combination. Pharm Chem J 56, 416–420 (2022). https://doi.org/10.1007/s11094-022-02644-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02644-9