Abstract
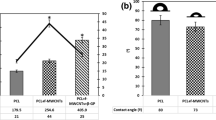
The integration of biomaterials in tissue regeneration has been showing effectiveness in the treatment of diseases related to bone structure and tissue repair. Membranes have aroused interest due to their ease of manufacture, variation in composition, and the structure of the biomaterial. The incorporation of bioactive glass (BG) increases bioactivity, and when doped with therapeutic ions, changes in the physical-chemical composition of the biomaterial are expected to enhance its biological effect. This study aimed to produce polycaprolactone (PCL) membranes incorporated with 58S bioactive glass, doped with Zinc (Zn) by the electrospinning technique, and evaluate the influence of this biomaterial in the activity and differentiation of mesenchymal stem cells. The BG was produced by using the sol-gel process; next, before the PCL preparation, the BG was doped with zinc in a solution. Then, PCL solutions were prepared with 7% by weight of BG and doped with 10% ZnCl2. Afterward, the electrospinning process was carried out using the fixed parameters: 2mLh-1 flow rate, 10kV voltage, and 12cm distance. Before the biological assays, the chemical elements present in the fibers were evaluated by energy dispersion X-ray spectroscopy (EDS), and the mapping technique. The morphology of the biomaterial and the diameter of fibers were analyzed by scanning electron microscopy (SEM), and the hydrophilicity of the membranes was evaluated by the contact angle technique. The in vitro tests consisted of cell plating with mesenchymal stem cells (MSC’s), previously obtained from rat femurs, at a density of 1x104 per well that contained three different groups: a) P: mesenchymal stem cells plated with PCL; b) PB: mesenchymal stem cells plated with the composite of PCL / BG; c) PBZ: mesenchymal stem cells plated with the Zn doped PCL / BG composite. To evaluate the influence of the biomaterial on osteoblastic activity and differentiation, osteogenic and non-osteogenic media were used in tests of cell viability (MTT assay), total protein content, alkaline phosphatase activity (ALP), and mineralization nodules. The analysis by SEM proved that the electrospinning technique was efficient for producing fibers incorporated with bioactive glass, and EDS and the mapping technique confirmed the chemical components of each group of fibers, including the doped zinc in the bioactive glass. The analysis of fibers diameter showed that P and PBZ had presented fibers with a larger diameter than the PB group, and the contact angle technique showed an increase in the hydrophilicity of the group containing doped Zinc when compared to the other groups analyzed. The MTT assay confirmed that the membranes weren´t cytotoxic and allowed cell viability, total protein content showed that all the groups had cell activity, with a statistically significant difference between the groups (p<0,05). Even with no statistically significant difference, osteogenesis was proved by ALP activity and the formation of mineralization nodules. Based on the results, the PCL membranes incorporated with 58S bioactive glass doped with zinc have shown promise in tissue engineering for use in bone tissue regeneration.









Similar content being viewed by others
References
De Moura NK, Martins EF, Oliveira RLMS, de Brito Siqueira IAW, Machado JPB, Esposito E, de Sousa Trichês E (2020) Synergistic effect of adding bioglass and carbon nanotubes on poly (lactic acid) porous membranes for guided bone regeneration. Mater Sci Eng: C. https://doi.org/10.1016/j.msec.2020.111327
Wang W, Yeung KWK (2017) Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioactive Mater 2(4):224–247. https://doi.org/10.1016/j.bioactmat.2017.05.0
Kim J-M, Lin C, Stavre Z, Greenblatt MB, Shim J-H (2020) Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 9(9):2073. https://doi.org/10.3390/cells9092073
Abdulghani S, Mitchell GR (2019) Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 9(11):750. https://doi.org/10.3390/biom9110750
Baroli B (2009) From natural bone grafts to tissue engineering therapeutics: Brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci 98(4):1317–1375. https://doi.org/10.1002/jps.21528
Chen J, Ashames A, Ali Buabeid M, Mustafa Fahelelbom K, Ijaz M, Murtaza G (2020) Nanocomposites drug delivery systems for the healing of bone fractures. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2020.119477
Doostmohammadi M, Forootanfar H, Ramakrishna S (2019) Regenerative medicine and drug delivery: Progress via electrospun biomaterials. Mater Sci Eng: C. https://doi.org/10.1016/j.msec.2019.110521
Elgali I, Omar O, Dahlin C, Thomsen P (2017) Guided bone regeneration: materials and biological mechanisms revisited. Eur J Oral Sci 125(5):315–337. https://doi.org/10.1111/eos.12364
Lee J, Byun H, Madhurakkat Perikamana SK, Lee S, Shin H (2018) Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv Healthc Mater. https://doi.org/10.1002/adhm.201801106
Rotman SG, Grijpma DW, Richards RG, Moriarty TF, Eglin D, Guillaume O (2018) Drug delivery systems functionalized with bone mineral seeking agents for bone targeted therapeutics. J Control Release 269:88–99. https://doi.org/10.1016/j.jconrel.2017.11.009
Zeng Y, Hoque J, Varghese S (2019) Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair. Acta Biomaterialia 93:152–168. https://doi.org/10.1016/j.actbio.2019.01.060
Zhao X (2011) Bioactive materials in orthopaedics. Bioactive Mater Med. https://doi.org/10.1533/9780857092939.2.124
Grandi G, Heitz C, dos Santos LA, Silva ML, Sant’Ana Filho M, Pagnocelli RM, Silva DN (2011) Comparative histomorphometric analysis between α-Tcp cement and β-Tcp/Ha granules in the bone repair of rat calvaria. Mater Res 14(1):11–16. https://doi.org/10.1590/s1516-14392011005000020
da Fonseca GF, Avelino SD, Mello DD, do Prado RF, Campos TM, de Vasconcellos LM, de Sousa Trichês E, Borges AL (2020) Scaffolds of PCL combined to bioglass: synthesis, characterization and biological performance. J Mater Sci: Mater Med 31(5). https://doi.org/10.1007/s10856-020-06382-w
Saghazadeh S, Rinoldi C, Schot M, Kashaf SS, Sharifi F, Jalilian E, Khademhosseini A (2018) Drug delivery systems and materials for wound healing applications. Adv Drug Deliv Rev 127:138–166. https://doi.org/10.1016/j.addr.2018.04.008
Rajzer I, Dziadek M, Kurowska A, Cholewa-Kowalska K, Ziąbka M, Menaszek E, Douglas TE (2019) Electrospun polycaprolactone membranes with Zn-doped bioglass for nasal tissues treatment. J Mater Sci: Mater Med 30(7). https://doi.org/10.1007/s10856-019-6280-4
Miszuk JM, Xu T, Yao Q, Fang F, Childs JD, Hong Z, Sun H (2018) Functionalization of PCL-3D electrospun nanofibrous scaffolds for improved BMP2-induced bone formation. Appl Mater Today 10:194–202. https://doi.org/10.1016/j.apmt.2017.12.004
Park YJ, Cha JH, Bang SI, Kim SY (2018) Clinical Application of Three-Dimensionally Printed Biomaterial Polycaprolactone (PCL) in Augmentation Rhinoplasty. Aesthet Plast Surg. https://doi.org/10.1007/s00266-018-1280-1
Granel H, Bossard C, Collignon A-M, Wauquier F, Lesieur J, Rochefort GY, Wittrant Y (2019) Bioactive Glass / Polycaprolactone Hybrid with Dual Cortical / Trabecular Structure for Bone Regeneration. ACS Appl Bio Mater. https://doi.org/10.1021/acsabm.9b00407
Anindyajati A, Boughton P, Ruys A (2017) Fabrication and Microstructure Evaluation of Fibrous Composite for Acetabular Labrum Implant. Mater Sci Forum 900:17–22. https://doi.org/10.4028/www.scientific.net/msf.900.17
Reneker DH, Yarin AL (2008) Electrospinning jets and polymer nanofibers. Polymer 49(10):2387–2425. https://doi.org/10.1016/j.polymer.2008.02.002
Iaquinta M, Mazzoni E, Manfrini M, D’Agostino A, Trevisiol L, Nocini R, Tognon M (2019) Innovative Biomaterials for Bone Regrowth. Int J Mol Sci 20(3):618. https://doi.org/10.3390/ijms20030618
Santana-Melo GF, Rodrigues BVM, da Silva E, Ricci R, Marciano FR, Webster TJ, Lobo AO (2017) Electrospun ultrathin PBAT/nHAp fibers influenced the in vitro and in vivo osteogenesis and improved the mechanical properties of neoformed bone. Colloids Surf B Biointerfaces 155:544–552. https://doi.org/10.1016/j.colsurfb.2017.04.053
Sato T, Mello D, Vasconcellos L, Valente A, Borges A (2020) Chitosan-Based Coacervate Polymers for Propolis Encapsulation: Release and Cytotoxicity Studies. Int J Mol Sci 21(12):4561. https://doi.org/10.3390/ijms21124561
Hench LL (2006) The story of Bioglass®. J Mater Sci: Mater Med 17(11):967–978. https://doi.org/10.1007/s10856-006-0432-z
Bellucci D, Chiellini F, Ciardelli G, Gazzarri M, Gentile P, Sola A, Cannillo V (2012) Processing and characterization of innovative scaffolds for bone tissue engineering. J Mater Sci: Mater Med 23(6):1397–1409. https://doi.org/10.1007/s10856-012-4622-6
Sista S, Nouri A, Li Y, Wen C, Hodgson PD, Pande G (2013) Cell biological responses of osteoblasts on anodized nanotubular surface of a titanium-zirconium alloy. J Biomed Mater Res Part A 101(12):3416–3430. https://doi.org/10.1002/jbm.a.34638
Rizwan M, Hamdi M, Basirun WJ (2017) Bioglass® 45S5-based composites for bone tissue engineering and functional applications. J Biomed Mater Res Part A 105(11):3197–3223. https://doi.org/10.1002/jbm.a.36156
Shi C, Wu T, He Y, Zhang Y, Fu D (2020) Recent advances in bone-targeted therapy. Pharmacol Ther. https://doi.org/10.1016/j.pharmthera.2020.107473
Mourino V, Cattalini JP, Boccaccini AR (2011) Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. J R Soc Interface 9(68):401–419. https://doi.org/10.1098/rsif.2011.0611
Münchow EA, Albuquerque MTP, Zero B, Kamocki K, Piva E, Gregory RL, Bottino MC (2015) Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration. Dental Mater 31(9):1038–1051. https://doi.org/10.1016/j.dental.2015.06.004
Vukajlovic D, Parker J, Bretcanu O, Novakovic K (2018) Chitosan based polymer/bioglass composites for tissue engineering applications. Mater Sci Eng: C. https://doi.org/10.1016/j.msec.2018.12.026
Neščáková Z, Kaňková H, Galusková D, Galusek D, Boccaccini AR, Liverani L (2021) Polymer (PCL) fibers with Zn-doped mesoporous bioactive glass nanoparticles for tissue regeneration. Int J Appl Glass Sci 12(4):588–600. https://doi.org/10.1111/ijag.16292
Yu W, Sun T-W, Qi C, Ding Z, Zhao H, Zhao S, He Y (2017) Evaluation of zinc-doped mesoporous hydroxyapatite microspheres for the construction of a novel biomimetic scaffold optimized for bone augmentation. Int J Nanomed 12:2293–2306. https://doi.org/10.2147/ijn.s126505
Dias AM, da Silva FG, de Figueiredo Monteiro AP, Pinzón-García AD, Sinisterra RD, Cortés ME (2019) Polycaprolactone nanofibers loaded oxytetracycline hydrochloride and zinc oxide for treatment of periodontal disease. Mater Sci Eng: C 103
Su Y, Cockerill I, Wang Y, Qin Y-X, Chang L, Zheng Y, Zhu D (2018) Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2018.10.009
Zanni E, Bruni E, Chandraiahgari CR, De Bellis G, Santangelo MG, Leone M, Bregnocchi A, Mancini P, Sarto MS, Uccelletti D (2017) Evaluation of the antibacterial power and biocompatibility of zinc oxide nanorods decorated graphene nanoplatelets: new perspectives for antibiodeteriorative approaches. J Nanobiotechnol 15(1). https://doi.org/10.1186/s12951-017-0291-4
Ingavle GC, Leach JK (2014) Advancements in Electrospinning of Polymeric Nanofibrous Scaffolds for Tissue Engineering. Tissue Eng Part B: Rev 20(4):277–293. https://doi.org/10.1089/ten.teb.2013.0276
Mei L, Wang Y, Tong A, Guo G (2016) Facile electrospinning of an efficient drug delivery system. Expert Op Drug Deliv 13(5):741–753. https://doi.org/10.1517/17425247.2016.1142525
Zelkó R, Lamprou DA, Sebe I (2019) Recent Development of Electrospinning for Drug Delivery. Pharmaceutics 12(1):5. https://doi.org/10.3390/pharmaceutics12010005
Zhu S, Liu Y, Gu Z, Zhao Y (2021) A Bibliometric Analysis of Advanced Healthcare Materials : Research Trends of Biomaterials in Healthcare Application. Adv Healthc Mater 10(10):2002222. https://doi.org/10.1002/adhm.202002222
Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, Shu W (2018) 3D bioactive composite scaffolds for bone tissue engineering. Bioactive Mater 3(3):278–314. https://doi.org/10.1016/j.bioactmat.2017.10.001
Wang L, Wang C, Wu S, Fan Y, Li X (2020) Influence of mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: current progress and challenges. Biomater Sci. https://doi.org/10.1039/d0bm00269k
Holzapfel BM, Reichert JC, Schantz J-T, Gbureck U, Rackwitz L, Nöth U, Hutmacher DW (2013) How smart do biomaterials need to be? A translational science and clinical point of view. Adv Drug Deliv Rev 65(4):581–603. https://doi.org/10.1016/j.addr.2012.07.009
Boudriot U, Dersch R, Greiner A, Wendorff JH (2006) Electrospinning Approaches Toward Scaffold Engineering: A Brief Overview. Artificial Organs, 30(10), 785–792. https://doi.org/10.1111/j.1525-1594.2006.00301.x
De Souza JR, Sato TP, Borges ALS (2017). Scaffold architecture for dental biomaterials: influence of process parameters on the structural morphology of chitosan electrospun fibers. Brazilian Dental Science 20(4). https://doi.org/10.14295/bds.2017.v20i4.1495
Calori IR, Braga G, Carvalho de Jesus P da C, Bi H, Tedesco, AC (2020) Polymer Scaffolds as Drug Delivery Systems. European Polymer Journal 109621. https://doi.org/10.1016/j.eurpolymj.2020.109621
Allafchian A, Jalali SAH, Mousavi SE, Hosseini SS (2019) Preparation of cell culture scaffolds using polycaprolactone/quince seed mucilage. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.11.09
Benhacine F, Abdellaoui N, Arous O, Hadj-Hamou A (2018) Behaviours Of Poly (Ε-Caprolactone) /Silver- Montmorillonite Nanocomposite In Membrane Ultrafiltration For Wastewater Treatment. Environ Technol. https://doi.org/10.1080/09593330.2018.1555283
Kukulka EC, Sato TP, Archangelo KC, Santos JD, De Souza JR, Borges ALS (2021) Development of electrospun-based polyetherimide fibers and diameter analysis for potential use in dental materials. Brazilian Dent Sci 24(2):1–5. https://doi.org/10.14295/bds.2021.v24i2.2107
Milleret V, Hefti T, Hall H, Vogel V, Eberli D (2012) Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation. Acta Biomaterialia 8(12):4349–4356. https://doi.org/10.1016/j.actbio.2012.07.032
Suarez-franco JL, Vázquez-Vázquez FC, Pozos-Guillen A, Montesinos JJ, Alvarez-Fregoso O, Alvarez-Perez MA (2018) Influence of diameter of fiber membrane scaffolds on the biocompatibility of hPDL mesenchymal stromal cells. Dental Mater J 37(3):465–473. https://doi.org/10.4012/dmj.2016-329
Pelipenko J, Kocbek P, Kristl J (2015) Nanofiber diameter as a critical parameter affecting skin cell response. European J Pharm Sci 66:29–35. https://doi.org/10.1016/j.ejps.2014.09.022
Spirandeli BR, Campos TMB, Ribas RG, Thim GP, Trichês ES (2020) Evaluation of colloidal and polymeric routes in sol-gel synthesis of a bioactive glass-ceramic derived from 45S5 bioglass. Ceramics Int. https://doi.org/10.1016/j.ceramint.2020.05.108
Pereira R do V (2013) Development of biodegradable 58s pldla/bioglass scaffolds produced by selective laser sintering. Dissertation (Master's in Materials Science and Engineering) - Federal University of Santa Catarina. Florianopolis 94
Zhang P, Yang K, Zhou Z, Zhu X, Li W, Cao C, Ai F (2020) Customized Borosilicate Bioglass Scaffolds With Excellent Biodegradation and Osteogenesis for Mandible Reconstruction. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.610284
Joughehdoust S, Manafi S (2012) Synthesis and in vitro investigation of sol-gel derived bioglass-58S nanopowders. Materials Science-Poland, 30(1), 45–52. https://doi.org/10.2478/s13536-012-0007-2
Ruan C, Hu N, Ma Y, Li Y, Liu J, Zhang X, Pan H (2017) The interfacial pH of acidic degradable polymeric biomaterials and its effects on osteoblast behavior. Scientific Rep 7(1). https://doi.org/10.1038/s41598-017-06354-1
Obata A, Iwanaga N, Terada A, Jell G, Kasuga T (2017) Osteoblast-like cell responses to silicate ions released from 45S5-type bioactive glass and siloxane-doped vaterite. J Mater Sci 52(15):8942–8956. https://doi.org/10.1007/s10853-017-1057-y
Liu W, Thomopoulos S, Xia Y (2011) Electrospun Nanofibers for Regenerative Medicine. Adv Healthc Mater 1(1):10–25. https://doi.org/10.1002/adhm.201100021
Mourino V, Boccaccini AR (2009) Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J Royal Soc Interf 7(43):209–227. https://doi.org/10.1098/rsif.2009.0379
Lowry O, Rosebrough N, Farr AL, Randall R (1951) Protein Measurement With The Folin Phenol Reagent. J Biol Chem 193(1):265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
Kim D-A, Lee J-H, Jun S-K, Kim H-W, Eltohamy M, Lee H-H (2017) Sol–gel-derived bioactive glass nanoparticle-incorporated glass ionomer cement with or without chitosan for enhanced mechanical and biomineralization properties. Dental Mater 33(7):805–817. https://doi.org/10.1016/j.dental.2017.04.017
Cai Q, Bei J, Wang S (2000) Synthesis and degradation of a tri-component copolymer derived from glycolide, L-lactide, and ε-caprolactone. J Biomater Sci Polym Ed 11(3):273–288. https://doi.org/10.1163/156856200743698
Gómez-Cerezo N, Casarrubios L, Saiz-Pardo M, Ortega L, de Pablo D, Díaz-Güemes I, Vallet-Regí M (2019) Mesoporous bioactive glass/ɛ-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. Acta Biomaterialia. https://doi.org/10.1016/j.actbio.2019.04.019
Jinga S-I, Costea C-C, Zamfirescu A-I, Banciu A, Banciu D-D, Busuioc C (2020) Composite Fiber Networks Based on Polycaprolactone and Bioactive Glass-Ceramics for Tissue Engineering Applications. Polymers 12(8):1806. https://doi.org/10.3390/polym12081806
Acknowledgements
We thank the São Paulo Research Foundation - FAPESP, for the scholarship process no. 2019/25744-9., 2020/12874-9 and 2020/12507-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernandes, M.S., Kukulka, E.C., de Souza, J.R. et al. Development and characterization of PCL membranes incorporated with Zn-doped bioactive glass produced by electrospinning for osteogenesis evaluation. J Polym Res 29, 370 (2022). https://doi.org/10.1007/s10965-022-03208-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03208-x