Abstract
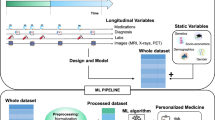
The incidence of systemic and metabolic co-morbidities increases with aging. The purpose was to investigate a novel paradigm for modeling the orchestrated changes in many disease-related biomarkers that occur during aging. A hybrid strategy that integrates machine learning and stochastic modeling was evaluated for modeling the long-term dynamics of biomarker systems. Bayesian networks (BN) were used to identify quantitative systems pharmacology (QSP)-like models for the inter-dependencies for three disease-related datasets of metabolic (MB), metabolic with leptin (MB-L), and cardiovascular (CVB) biomarkers from the NHANES database. Biomarker dynamics were modeled using discrete stochastic vector autoregression (VAR) equations. BN were used to derive the topological order and connectivity of a data driven QSP model structure for inter-dependence of biomarkers across the lifespan. The strength and directionality of the connections in the QSP models were evaluated using bootstrapping. VAR models based on QSP model structures from BN were assessed for modeling biomarker system dynamics. BN-restricted VAR models of order 1 were identified as parsimonious and effective for characterizing biomarker system dynamics in the MB, MB-L and CVB datasets. Simulation of annual and triennial data for each biomarker provided good fits and predictions of the training and test datasets, respectively. The novel strategy harnesses machine learning to construct QSP model structures for inter-dependence of biomarkers. Stochastic modeling with the QSP models was effective for predicting the age-varying dynamics of disease-relevant biomarkers over the lifespan.




Similar content being viewed by others
References
World Health Organization (2018) Ageing and health. World Health Organization, Geneva
National Institute of Aging (2020) The National Institute On Aging: Strategic Directions For Research, 2020–2025: understanding the dynamics of the aging process. U.S, Department of Health and Human Services, Bethesda
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217
Mangoni AA, Jackson SH (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 57(1):6–14
Shi S, Morike K, Klotz U (2008) The clinical implications of ageing for rational drug therapy. Eur J Clin Pharmacol 64(2):183–199
Trifiro G, Spina E (2011) Age-related changes in pharmacodynamics: focus on drugs acting on central nervous and cardiovascular systems. Curr Drug Metab 12(7):611–620
Masoro EJ (1988) Physiological system markers of aging. Exp Gerontol 23(4–5):391–394
NIH. Understanding the dynamics of the aging process. NIH, Bethesda
Strimbu K, Tavel JA (2010) What are biomarkers? Curr Opin HIV AIDS 5(6):463–466
Holford N (2015) Clinical pharmacology = disease progression + drug action. Br J Clin Pharmacol 79(1):18–27
Holford N (2019) Treatment response and disease progression. Transl Clin Pharmacol 27(4):123–126
Peterson MC, Riggs MM (2010) A physiologically based mathematical model of integrated calcium homeostasis and bone remodeling. Bone 46(1):49–63
Landersdorfer CB, Jusko WJ (2008) Pharmacokinetic/pharmacodynamic modelling in diabetes mellitus. Clin Pharmacokinet 47(7):417–448
Lon HK, Liu D, Zhang Q, DuBois DC, Almon RR, Jusko WJ (2011) Pharmacokinetic-pharmacodynamic disease progression model for effect of etanercept in Lewis rats with collagen-induced arthritis. Pharm Res 28(7):1622–1630
McComb M, Bies R, Ramanathan M (2021) Machine learning in pharmacometrics: opportunities and challenges. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.14801
Centers for Disease Control and Prevention (CDC) (2012) Principles of epidemiology in public health practice. United States Department of Heallth and Human Services, Washington DC
Talevi A, Morales JF, Hather G, Podichetty JT, Kim S, Bloomingdale PC et al (2020) Machine learning in drug discovery and development part 1: a primer. CPT Pharmacometrics Syst Pharmacol 9(3):129–142
Chaturvedula A, Calad-Thomson S, Liu C, Sale M, Gattu N, Goyal N (2019) Artificial intelligence and pharmacometrics: time to embrace, capitalize, and advance? CPT Pharmacometrics Syst Pharmacol 8(7):440–443
Chow HH, Tolle KM, Roe DJ, Elsberry V, Chen H (1997) Application of neural networks to population pharmacokinetic data analysis. J Pharm Sci 86(7):840–845
McComb M, Ramanathan M (2020) Generalized pharmacometric modeling, a novel paradigm for integrating machine learning algorithms: a case study of metabolomic biomarkers. Clin Pharmacol Ther 107(6):1343–1351
Crimmins E, Vasunilashorn S, Kim JK, Alley D (2008) Biomarkers related to aging in human populations. Adv Clin Chem 46:161–216
National Center for Health Statistics (2018) National health and nutrition survey: NHANES III (1988–1994). Centers for Disease Control and Prevention, Hyattsville
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Ishwaran H, Kogalur U (2020) Fast unified random forests for survival, regression, and classification (RF-SRC). 2.9.3 ed2020. p. R package
Hong S, Lynn HS (2020) Accuracy of random-forest-based imputation of missing data in the presence of non-normality, non-linearity, and interaction. BMC Med Res Methodol 20(1):199
Koller D, Friedman N (2009) Probabilistic graphical models: principles and techniques. MIT, Cambridge
Scutari M (2010) Learning Bayesian networks with the bnlearn R package. J Stat Softw 35(3):1–22
Granger CWJ (1969) Investigating causal relations by econometric models and cross-spectral methods. Econometrica 37(3):424–438
Lavielle M (2018) Pharmacometrics models with hidden Markovian dynamics. J Pharmacokinet Pharmacodyn 45(1):91–105
Ghahramani Z (1998) Learning dynamic Bayesian networks. In: Giles CL, Gori M (eds) Adaptive processing of sequences and data structures: international summer school on neural networks “ER Caianiello” Vietri sul Mare, Salerno, Italy, 6–13 September 1997, Tutorial Lectures. Springer, Berlin, pp 168–197
Lauritzen SL (1996) Graphical models. Clarendon Press/Oxford University Press, Oxford/New York
Pearl J (2000) Causality: models, reasoning, and inference. Cambridge University Press, Cambridge
Fellows K, Stoneking CJ, Ramanathan M (2015) Bayesian population modeling of drug dosing adherence. J Pharmacokinet Pharmacodyn 42(5):515–525
Knights J, Heidary Z, Peters-Strickland T, Ramanathan M (2019) Evaluating digital medicine ingestion data from seriously mentally ill patients with a Bayesian Hybrid Model. NPJ Digit Med 2:20
Sims CA, Stock JH, Watson MW (1990) Inference in linear time series models with some unit roots. Econometrica 58(1):113–144
Engle RF, Granger CWJ (1987) Co-integration and error correction: representation, estimation and testing. Econometrica 55(2):251–276
Acknowledgements
Funding for the Ramanathan laboratory from MS190096 from Department of Defense Congressionally Directed Medical Research Programs, USAMRDC, Multiple Sclerosis Research Program and from NIGMS 131800 (PI: William Jusko) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
MM—performed research, analyzed data, wrote manuscript. RHB—data interpretation, Bayesian networks, manuscript preparation. ML—stochastic modeling, manuscript preparation. MR—study concept and design, data analysis and interpretation, manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest:
Mason McComb has no conflicts to disclose. Rachael Hageman Blair received funding from Department of Defense. Martin Lysy has no conflicts to disclose. Murali Ramanathan received research funding from the National Science Foundation, Department of Defense and Otsuka Pharmaceuticals and the National Institutes of Health. These are unrelated to the research presented in this report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McComb, M., Blair, R.H., Lysy, M. et al. Machine learning-guided, big data-enabled, biomarker-based systems pharmacology: modeling the stochasticity of natural history and disease progression. J Pharmacokinet Pharmacodyn 49, 65–79 (2022). https://doi.org/10.1007/s10928-021-09786-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-021-09786-5