Abstract
Antisense oligonucleotides (ASOs) are promising therapeutic agents for a variety of neurodegenerative and neuromuscular disorders, e.g., Alzheimer’s, Parkinson’s and Huntington’s diseases, spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS), caused by genetic abnormalities or increased protein accumulation. The blood–brain barrier (BBB) represents a challenge to the delivery of systemically administered ASOs to the relevant sites of action within the central nervous system (CNS). Intrathecal (IT) delivery, in which drugs are administered directly into the cerebrospinal fluid (CSF) space, enables to bypass the BBB. Several IT-administered ASO therapeutics have already demonstrated clinical effect, e.g., nusinersen (SMA) and tofersen (ALS). Due to novelty of IT dosing for ASOs, very limited pharmacokinetic (PK) data is available and only a few modeling reports have been generated. The objective of this work is to advance fundamental understanding of whole-body distribution of IT-administered ASOs. We propose a physiologically-based pharmacokinetic modeling approach to describe the distribution along the neuroaxis based on PK data from non-human primate (NHP) studies. We aim to understand the key processes that drive and limit ASO access to the CNS target tissues. To elucidate the trade-off between parameter identifiability and physiological plausibility of the model, several alternative model structures were chosen and fitted to the NHP data. The model analysis of the NHP data led to important qualitative conclusions that can inform projection to human. In particular, the model predicts that the maximum total exposure in the CNS tissues, including the spinal cord and brain, is achieved within two days after the IT injection, and the maximum amount absorbed by the CNS tissues is about 4% of the administered IT dose. This amount greatly exceeds the CNS exposures delivered by systemic administration of ASOs. Clearance from the CNS is controlled by the rate of transfer from the CNS tissues back to CSF, whereas ASO degradation in tissues is very slow and can be neglected. The model also describes local differences in ASO concentration emerging along the spinal CSF canal. These local concentrations need to be taken into account when scaling the NHP model to human: due to the lengthier human spinal column, inhomogeneity along the spinal CSF may cause even higher gradients and delays potentially limiting ASO access to target CNS tissues.






Similar content being viewed by others
References
Calias P, Banks WA, Begley D, Scarpa M, Dickson P (2014) Intrathecal delivery of protein therapeutics to the brain. Pharm Ther 144:114–122
Thorne RG, Frey WH (2001) Delivery of neurotropic factors to the central nervous system. Pharmacokinetic considerations. Clin Pharmacokinet 40:907–946
Ummenhofer WC, Arends RH, Shen DD, Bernards CM (2000) Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanyl. Anesthesiology 92:739–753
Chiriboga CA, Swoboda KJ, Darras BT, Iannaccone ST, Montes J, De Vivo DC, Norris DA, Bennett CF, Bishop KM (2016) Results from a phase 1 study of nusinersen (ISIS-SMNRx) in children with spinal muscular atrophy. Neurology 86:890–897
Finkel RS, Chiriboga CA, Vajsar J, Day JW, Montes J, De Vivo DC, Yamashita M, Rigo F, Hung G, Schneider E, Norris DA, Xia S, Bennett CF, Bishop KM (2016) Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388:3017–3026
Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, Wild EJ, Saft C, Barker RA, Blair NF, Craufurd D, Priller J, Rickards H, Rosser A, Kordasiewicz HB, Czech C, Swayze EE, Norris DA, Baumann T, Gerlach I, Schobel SA, Paz E, Smith AV, Bennett CF, Lane RM (2019) Targeting huntingtin expression in patients with Huntington’s disease. N Engl J Med 380:2307–2316
Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, Chiriboga CA, Saito K, Servais L, Tizzano E, Topaloglu H, Tulinius M, Montes J, Glanzman AM, Bishop K, Zhong ZH, Gheuens S, Bennett CF, Schneider E, Farwell W, De Vivo DC (2017) Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 377:1723–1732
Miller T, Cudkowicz M, Shaw PJ, Andersen PM, Atassi N, Bucelli RC, Genge A, Glass J, Ladha S, Ludolph AL, Maragakis NJ, McDermott CJ, Pestronk A, Ravits J, Salachas F, Trudell R, Van Damme P, Zinman L, Bennett CF, Lane R, Sandrock A, Runz H, Graham D, Houshyar H, McCampbell A, Nestorov I, Chang I, McNeill M, Fanning L, Fradette S, Ferguson TA (2020) Phase 1–2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med 383:109–119
Clemens PR, Rao VK, Connolly AM, Harper AD, Mah JK, Smith EC, McDonald CM, Zaidman CM, Morgenroth LP, Osaki H, Satou Y, Yamashita T, Hoffman EP (2020) Safety, tolerability, and efficacy of viltolarsen in boys with duchenne muscular dystrophy amenable to exon 53 skipping. a phase 2 randomized clinical trial. JAMA Neurol 77:1–10
Bennett CF, Krainer AR, Cleveland DW (2019) Antisense oligonucleotide therapies for neurodegenerative diseases. Annu Rev Neurosci 42:385–406
Geary RS, Norris D, Yu R, Bennett CF (2015) Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 87:46–51
Miller CM, Tanowitz M, Donner AJ, Prakash TP, Swayze EE, Harris EN, Seth PP (2018) Receptor-mediated uptake of phosphorothioate antisense oligonucleotides in different cell types of the liver. Nucleic Acid Ther 28:119–127
Rigo F, Chun SJ, Norris DA, Hung G, Lee S, Matson J, Fey RA, Gaus H, Hua Y, Grundy JS, Krainer AR, Henry SP, Bennett CF (2014) Pharmacology of a central nervous system delivered 2’-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J of Pharmacol Exp Ther 350:46–55
Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, Andres PL, Mahoney K, Allred P, Alexander K, Ostrow LW, Schoenfeld D, Macklin EA, Norris DA, Manousakis G, Crisp M, Smith R, Bennett CF, Bishop KM, Cudkowicz ME (2013) An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol 12:435–442
Evers MM, Toonen LJA, van Roon-Mom WMC (2015) Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv Drug Deliv Rev 87:90–103
Pardridge WM (2016) CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv 13:963–975
Hsu Y, Madhawa Hettiarachchi HD, Zhu DC, Linninger AA (2012) The frequency and magnitude of cerebrospinal fluid pulsations influence intrathecal drug distribution: key factors for interpatient variability. Anesth Analg 115:386–394
Laitinen L (1968) Origin of arterial pulsation of cerebrospinal fluid. Acta Neurol Scand 44:168–176
Krupp JL, Bernards CM (2004) Pharmacokinetics of intrathecal oligodeoxynucleotides. Anesthesiology 100:315–322
Biliouris K, Gaitonde P, Yin W, Norris DA, Wang Y, Henry S, Fey R, Nestorov I, Schmidt S, Rogge M, Lesko LJ, Trame MN (2018) A semi-mechanistic population pharmacokinetic model of nusinersen: an antisense oligonucleotide for the treatment of spinal muscular atrophy. CPT Pharmacometrics Syst Pharmacol 7:581–592
Luu KT, Norris DA, Gunawan R, Henry S, Geary R, Wang YJ (2017) Population pharmacokinetics of nusinersen in the cerebral spinal fluid and plasma of pediatric patients with spinal muscular atrophy following intrathecal administrations. Clin Pharmacol 57:1031–1041
Nestorov I (2007) Whole-body physiologically based pharmacokinetic models. Expert Opin Drug Metab Toxicol 3:235–249
Yamamoto Y, Välitalo PA, Huntjens DR, Proost JH, Vermeulen A, Krauwinkel W, Beukers MW, van den Berg DJ, Hartman R, Wong YC, Danhof M, van Hasselt JGC, de Lange ECM (2017) Predicting drug concentration-time profiles in multiple CNS compartments using a comprehensive physiologically-based pharmacokinetic model. CPT Pharmacometrics Syst Pharmacol 6:765–777
Peng B, Andrews J, Nestorov I, Brennan B, Nicklin P, Rowland M (2001) Tissue distribution and physiologically based pharmacokinetics of antisense phosphorothioate oligonucleotide ISIS 1082 in rat. Antisense Nucleic Acid Drug Dev 11:15–27
Chang HY, Wu S, Meno-Tetang G, Shah DK (2019) A translational platform PBPK model for antibody disposition in the brain. J Pharmacokinet Pharmacodyn 46:319–338
Yu RZ, Kim TW, Hong A, Watanabe TA, Gaus HJ, Geary RS (2007) Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab Dispos 35:460–468
Yu RZ, Grundy JS, Henry SP, Kim TW, Norris DA, Burkey J, Wang Y, Vick A, Geary RS (2015) Predictive dose-based estimation of systemic exposure multiples in mouse and monkey relative to human for antisense oligonucleotides with 2’-o-(2-methoxyethyl) modifications. Mol Ther Nucleic Acids 4:e218
Scoles DR, Pulst SM (2019) Antisense therapies for movement disorders. Mov Disord 34:1112–1119
Pardo ID, Garman RH, Weber K, Bobrowski WF, Hardisty JF, Morton D (2012) Technical guide for nervous system sampling of the cynomolgus monkey for general toxicity studies. Toxicol Pathol 40:624–636
Sakamoto K, Sawada K, Fukunishi K, Noritaka I, Sakata-Haga H, Yoshihiro F (2014) Postnatal change in sulcal length asymmetry in cerebrum of cynomolgus monkeys (Macaca fascicularis). Anat Rec 297:200–207
Ageyama N, Shibata H, Narita H, Hanari K, Kohno A, Ono F, Yoshikawa Y, Terao K (2001) Specific gravity of whole blood in cynomolgus monkeys (Macaca fascicularis), squirrel monkeys (Saimiri sciureus), and tamarins (Saguinus labiatus) and total blood volume in cynomolgus monkeys. Contemp Topics Lab Anim Sci/Am Assoc Lab Anim Sci 40:33–35
Geary RS, Yu RZ, Watanabe T, Henry SP, Hardee GE, Chappell A, Matson J, Sasmor H, Cummins L, Levin AA (2003) Pharmacokinetics of a tumor necrosis factor-α phosphorothioate 2′-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug Metab Dispos 31:1419–1428
Yu RZ, Baer B, Chappel A, Geary RS, Chueng E, Levin AA (2002) Development of an ultrasensitive noncompetitive hybridization-ligation enzyme-linked immunosorbent assay for the determination of phosphorothioate oligodeoxynucleotide in plasma. Anal Biochem 304:19–25
Yu RZ, Geary RS, Levin AA (2004) Application of novel quantitative bioanalytical methods for pharmacokinetic and pharmacokinetic/pharmacodynamic assessment of antisense oligonucleotides. Curr Opin Drug Discov Devel 7:195–203
D’Argenio DZ, Schumitzky A, Wang X (2009) ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles
Di Chiro G, Knop RH, Girton ME, Dwyer AJ, Doppman JL, Patronas NJ, Gansow OA, Brechbiel MW, Brooks RA (1985) MR cisternography and myelography with Gd-DTPA in monkeys. Radiology 157:373–377
Sultan F, Braitenberg V (1993) Shapes and sizes of different mammalian cerebella. A study in quantitative comparative neuroanatomy. J Hirnforsch 34:79–92
Acknowledgements
We thank Dr. Alex McCampbell, Dr. Kumar Kandadi Muralidharan, Dr. Natasha Penner and Dr. Eric Masson for scientific discussions during model development and manuscript submission. We also thank Dr. Jeannette Stankowski for editing the manuscript, and anonymous reviewers for providing critical and valuable comments. Previously unpublished NHP data used in this work was generated by Ionis Pharmaceuticals. This work was funded by Biogen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix
Appendix
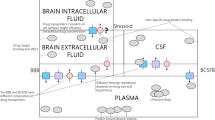
All models analyzed consist of a system of ordinary differential equations (ODEs) that are parameterized in terms of amounts. As defined in the equations below and in accordance with the schematics presented in Fig. 1, the amount of ASO in region \(i\) (e.g., CSF or tissue type) and specific location \(l\) is denoted by variable \(A_{i}^{l}\) that has units of mass (e.g., ng).
The two model modifications presented here share the same equations and parameter definitions but differ in the number of parameters to be estimated. Model 1 contains 32 parameters to be estimated, whereas in Model 2, the number of parameters to be estimated is reduced to 21 respectively. The following parameter values of Model 1 are lumped into single values in Model 2:
The following equations define the CNS structure in both models:
Systemic part equations:
Rights and permissions
About this article
Cite this article
Monine, M., Norris, D., Wang, Y. et al. A physiologically-based pharmacokinetic model to describe antisense oligonucleotide distribution after intrathecal administration. J Pharmacokinet Pharmacodyn 48, 639–654 (2021). https://doi.org/10.1007/s10928-021-09761-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-021-09761-0