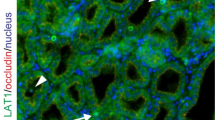
Abstract
Short-chain fatty acids activate antimicrobial component production in the intestine. However, their effects on mammary glands remain unclear. We investigated the effects of acetate and butyrate on antimicrobial component production in mammary epithelial cells (MECs) or leukocytes cultured in vitro and in mammary glands of lactating Tokara goats in vivo. Our results showed that butyrate enhanced the production of β-defensin-1 and S100A7 in MECs. Additionally, the infusion of butyrate into mammary glands through the teats enhanced β-defensin-1 and S100A7 concentrations in milk. The infusion of acetate also increased β-defensin-1 and S100A7 concentrations along with those of cathelicidin-2 and interleukin-8, which are produced by leukocytes. Furthermore, acetate promoted cathelicidin-2 and interleukin-8 secretion in leukocytes in vitro. These findings suggest that acetate and butyrate differentially upregulate antimicrobial component production in mammary glands, which could help to develop appropriate treatment for mastitis, thereby reducing economic losses and improving animal welfare in farming environments.







Similar content being viewed by others
References
Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–90.
Schwab M, Reynders V, Loitsch S, Steinhilber D, et al. The dietary histone deacetylase inhibitor sulforaphane induces human beta-defensin-2 in intestinal epithelial cells. Immunology. 2008;125:241–51.
Zhao Y, Chen F, Wu W, Sun M, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752–62.
Isobe N. Control mechanisms for producing antimicrobial factors in ruminant mammary gland. Anim Sci J. 2017;88:937–43.
Zarzosa-Moreno D, Avalos-Gomez C, Ramirez-Texcalco LS, Torres-Lopez E, et al., Lactoferrin and Its Derived Peptides: An Alternative for Combating Virulence Mechanisms Developed by Pathogens. Molecules 2020, 25.
Tang YQ, Yuan J, Osapay G, Osapay K, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502.
Chanu KV, Thakuria D, Kumar S. Antimicrobial peptides of buffalo and their role in host defenses. Vet World. 2018;11:192–200.
Wheeler TT, Smolenski GA, Harris DP, Gupta SK, et al. Host-defence-related proteins in cows’ milk. Animal. 2012;6:415–22.
Tomasinsig L, De Conti G, Skerlavaj B, Piccinini R, et al. Broad-spectrum activity against bacterial mastitis pathogens and activation of mammary epithelial cells support a protective role of neutrophil cathelicidins in bovine mastitis. Infect Immun. 2010;78:1781–8.
Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20.
Yeung AT, Gellatly SL, Hancock RE. Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci. 2011;68:2161–76.
Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–40.
Gunther J, Petzl W, Zerbe H, Schuberth HJ, et al. Lipopolysaccharide priming enhances expression of effectors of immune defence while decreasing expression of pro-inflammatory cytokines in mammary epithelia cells from cows. BMC Genomics. 2012;13:17.
Zhang GW, Lai SJ, Yoshimura Y, Isobe N. Expression of cathelicidins mRNA in the goat mammary gland and effect of the intramammary infusion of lipopolysaccharide on milk cathelicidin-2 concentration. Vet Microbiol. 2014;170:125–34.
Zhang GW, Lai SJ, Yoshimura Y, Isobe N. Messenger RNA expression and immunolocalization of psoriasin in the goat mammary gland and its milk concentration after an intramammary infusion of lipopolysaccharide. Vet J. 2014;202:89–93.
Purba FY, Nii T, Yoshimura Y, Isobe N. Short communication: Production of antimicrobial peptide S100A8 in the goat mammary gland and effect of intramammary infusion of lipopolysaccharide on S100A8 concentration in milk. J Dairy Sci. 2019;102:4674–81.
Tsugami Y, Matsunaga K, Suzuki T, Nishimura T, Kobayashi K. Isoflavones and their metabolites influence the milk component synthesis ability of mammary epithelial cells through prolactin/STAT5 signaling. Mol Nutr Food Res 2017, 61.
Tsugami Y, Suzuki N, Kawahara M, Suzuki T, et al. Establishment of an in vitro culture model to study milk production and the blood-milk barrier with bovine mammary epithelial cells. Anim Sci J. 2020;91:e13355.
Purba FY, Ueda J, Nii T, Yoshimura Y, Isobe N. Effects of intrauterine infusion of bacterial lipopolysaccharides on the mammary gland inflammatory response in goats. Vet Immunol Immunopathol. 2020;219:109972.
Kuwahara K, Yoshimura Y, Isobe N. Effect of steroid hormones on the innate immune response induced by Staphylococcus aureus in the goat mammary gland. Reprod Domest Anim. 2017;52:579–84.
Zhao Y, Yan S, Chen L, Shi B, Guo X. Effect of interaction between leucine and acetate on the milk protein synthesis in bovine mammary epithelial cells. Anim Sci J. 2019;90:81–9.
Chen J, Wu Y, Sun Y, Dong X, et al. Bacterial endotoxin decreased histone H3 acetylation of bovine mammary epithelial cells and the adverse effect was suppressed by sodium butyrate. BMC Vet Res. 2019;15:267.
Sharmin MM, Mizusawa M, Hayashi S, Arai W, et al. Effects of fatty acids on inducing endoplasmic reticulum stress in bovine mammary epithelial cells. J Dairy Sci. 2020;103:8643–54.
Xiong H, Guo B, Gan Z, Song D, et al. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci Rep. 2016;6:27070.
Dou X, Han J, Song W, Dong N, et al. Sodium butyrate improves porcine host defense peptide expression and relieves the inflammatory response upon Toll-like receptor 2 activation and histone deacetylase inhibition in porcine kidney cells. Oncotarget. 2017;8:26532–51.
Chen JS, Faller DV, Spanjaard RA. Short-chain fatty acid inhibitors of histone deacetylases: promising anticancer therapeutics? Curr Cancer Drug Targets. 2003;3:219–36.
Dou X, Gao N, Lan J, Han J, et al. TLR2/EGFR Are Two Sensors for pBD3 and pEP2C Induction by Sodium Butyrate Independent of HDAC Inhibition. J Agric Food Chem. 2020;68:512–22.
Tsugami Y, Wakasa H, Kawahara M, Watanabe A, et al. Adverse effects of LPS on membrane proteins in lactating bovine mammary epithelial cells. Cell Tissue Res. 2021;384:435–48.
Isobe N, Hosoda K, Yoshimura Y. Immunolocalization of lingual antimicrobial peptide (LAP) in the bovine mammary gland. Anim Sci J. 2009;80:446–50.
Wang A, Gu Z, Heid B, Akers RM, Jiang H. Identification and characterization of the bovine G protein-coupled receptor GPR41 and GPR43 genes. J Dairy Sci. 2009;92:2696–705.
Yonezawa T, Haga S, Kobayashi Y, Katoh K, Obara Y. Short-chain fatty acid signaling pathways in bovine mammary epithelial cells. Regul Pept. 2009;153:30–6.
Guo W, Liu J, Sun J, Gong Q, et al. Butyrate alleviates oxidative stress by regulating NRF2 nuclear accumulation and H3K9/14 acetylation via GPR109A in bovine mammary epithelial cells and mammary glands. Free Radic Biol Med. 2020;152:728–42.
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–9.
Dahlstrand Rudin A, Khamzeh A, Venkatakrishnan V, Basic A, et al. Short chain fatty acids released by Fusobacterium nucleatum are neutrophil chemoattractants acting via free fatty acid receptor 2 (FFAR2). Cell Microbiol. 2021;23:e13348.
Schlatterer K, Beck C, Schoppmeier U, Peschel A, Kretschmer D. Acetate sensing by GPR43 alarms neutrophils and protects from severe sepsis. Commun Biol. 2021;4:928.
Madsen P, Rasmussen HH, Leffers H, Honore B, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J Invest Dermatol. 1991;97:701–12.
Hagens G, Masouye I, Augsburger E, Hotz R, et al. Calcium-binding protein S100A7 and epidermal-type fatty acid-binding protein are associated in the cytosol of human keratinocytes. Biochem J. 1999;339(Pt 2):419–27.
Ruse M, Broome AM, Eckert RL. S100A7 (psoriasin) interacts with epidermal fatty acid binding protein and localizes in focal adhesion-like structures in cultured keratinocytes. J Invest Dermatol. 2003;121:132–41.
Ali I, Li C, Li L, Kuang M, et al. Effect of acetate, beta-hydroxybutyrate and their interaction on lipogenic gene expression, triglyceride contents and lipid droplet formation in dairy cow mammary epithelial cells. In Vitro Cell Dev Biol Anim. 2021;57:66–75.
Niyonsaba F, Hattori F, Maeyama K, Ogawa H, Okamoto K. Induction of a microbicidal protein psoriasin (S100A7), and its stimulatory effects on normal human keratinocytes. J Dermatol Sci. 2008;52:216–9.
Yumine A, Tsuji G, Furue M. Selective PPARalpha agonist pemafibrate inhibits TNF-alpha-induced S100A7 upregulation in keratinocytes. J Dermatol Sci. 2020;99:69–72.
Oikonomou G, Addis MF, Chassard C, Nader-Macias MEF, et al., Milk Microbiota: What Are We Exactly Talking About? Front Microbiol 2020, 11, 60.
Zhang S, Wang R, Li D, Zhao L, Zhu L. Role of gut microbiota in functional constipation. Gastroenterol Rep (Oxf). 2021;9:392–401.
Yang P, Zhao J. Variations on gut health and energy metabolism in pigs and humans by intake of different dietary fibers. Food Sci Nutr. 2021;9:4639–54.
Sun X, Luo S, Jiang C, Tang Y, et al. Sodium butyrate reduces bovine mammary epithelial cell inflammatory responses induced by exogenous lipopolysaccharide, by inactivating NF-kappaB signaling. J Dairy Sci. 2020;103:8388–97.
Ochoa-Zarzosa A, Villarreal-Fernandez E, Cano-Camacho H, Lopez-Meza JE. Sodium butyrate inhibits Staphylococcus aureus internalization in bovine mammary epithelial cells and induces the expression of antimicrobial peptide genes. Microb Pathog. 2009;47:1–7.
Acknowledgements
We thank Yukinori Yoshimura from the Laboratory of Animal Histophysiology, Graduate School of Integrated Sciences for Life, Hiroshima University, for helpful advice on this study.
Author information
Authors and Affiliations
Contributions
Yusaku Tsugami: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Validation, Writing – Original Draft. Naoki Suzuki: Conceptualization, Writing – Review & Editing. Takahiro Nii: Conceptualization, Resources, Writing – Review & Editing. Naoki Isobe: Conceptualization, Project administration, Supervision, Methodology, Resources, Writing – Review & Editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethical Approval
All experiments were approved by the Animal Research Committee of Hiroshima University (no. C21-20).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tsugami, Y., Suzuki, N., Nii, T. et al. Sodium Acetate and Sodium Butyrate Differentially Upregulate Antimicrobial Component Production in Mammary Glands of Lactating Goats. J Mammary Gland Biol Neoplasia 27, 133–144 (2022). https://doi.org/10.1007/s10911-022-09519-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-022-09519-5