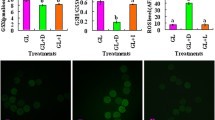
Abstract
Purpose
Glucose and redox metabolism characterization in mouse antral follicles with meiotically blocked oocytes, after in vitro follicle culture (IFC) from the early secondary stage.
Methods
Following IFC (10 days), oocytes, corresponding cumulus (CC), and granulosa cells (GC) were collected from antral follicles: (i) on day 9—immature, germinal vesicle (GV) stage; (ii) on day 10, after hCG/EGF stimulation—mature, metaphase II (MII) stage and meiotically blocked (MB) immature GV stage. The metabolic profiles of all samples (GV, MII, and MB) were compared by measuring changes in metabolites involved in glycolysis, tricarboxylic acid (TCA) cycle, pentose phosphate pathway (PPP), and redox activity via enzymatic spectrophotometric assays in each cell type.
Results
Within MB follicles, GCs drive higher levels of glycolysis and lactic acid fermentation (LAF) while oocytes exert more PPP activity. MB-oocytes had significantly larger diameters compared to day 9 GVs. MB follicles revealed limited metabolic changes in the somatic compartment compared to their GV counterparts (before stimulation). MB-CCs showed increased aconitase and glucose-6-phosphate dehydrogenase activities with lower malate levels comparted to GV-CCs. MB and MII in vitro grown follicles displayed comparable metabolic profiles, suggesting culture induces metabolic exhaustion regardless of the maturation stage.
Conclusions
Current results suggest that in addition to impaired nuclear maturation, metabolic disruption is present in MB follicles. MB follicles either compensate with high levels of TCA cycle and PPP activities in CCs, or are unable to drive proper levels of aerobic metabolism, which might be due to the current culture conditions.






Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9:1300.
De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. The Lancet. 2014;384:1302–10.
Telfer EE. Progress and prospects for developing human immature oocytes in vitro. Reproduction. 2019;158:F45–54.
Herta AC, Lolicato F, Smitz JEJ. In vitro follicle culture in the context of IVF. Reproduction. 2018;156(1):F59–73.
Cortvrindt S. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8:243–54.
Sánchez F, Romero S, Albuz FK, Smitz J. In vitro follicle growth under non-attachment conditions and decreased FSH levels reduces Lhcgr expression in cumulus cells and promotes oocyte developmental competence. J Assist Reprod Genet. 2012;29:141–52.
Saenz-De-Juano MD, Ivanova E, Billooye K, Herta A-C, Smitz J, Kelsey G, et al. Genome-wide assessment of DNA methylation in mouse oocytes reveals effects associated with in vitro growth, superovulation, and sexual maturity. Clin Epigenetics. 2019;11:197.
Segers I, Adriaenssens T, Ozturk E, Smitz J. Acquisition and loss of oocyte meiotic and developmental competence during in vitro antral follicle growth in mouse. Fertil Steril. 2010;93:2695–700.
Sánchez F, Adriaenssens T, Romero S, Smitz J. Different follicle-stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice1. Biol Reprod. 2010;83:514–24.
Saenz-de-Juano MD, Billooye K, Smitz J, Anckaert E. The loss of imprinted DNA methylation in mouse blastocysts is inflicted to a similar extent by in vitro follicle culture and ovulation induction. Mol Hum Reprod. 2016;22:427–41.
Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139(4):685–95.
Herta A-C, von Mengden L, Akin N, Billooye K, Coucke W, van Leersum J, et al. Characterization of carbohydrate metabolism in in vivo- and in vitro-grown and matured mouse antral follicles. Biol Reprod. 2022;107(4):998–1013.
Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol. 1976;50:531–6.
Sirard MA, Richard F, Blondin P, Robert C. Contribution of the oocyte to embryo quality. Theriogenology. 2006;65(1):126–36.
Leese HJ, Lenton EA. Glucose and lactate in human follicular fluid: concentrations and interrelationships. Hum Reprod. 1990;5(8):915–9.
Redding GP, Bronlund JE, Hart AL. Mathematical modelling of oxygen transport-limited follicle growth. Reproduction. 2007;133:1095–106.
Harris SE, Gopichandran N, Picton HM, Leese HJ, Orsi NM. Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology. 2005;64:992–1006.
Lim M, Thompson JG, Dunning KR. Hypoxia and ovarian function: follicle development, ovulation, oocyte maturation. REP. 2021;161:F33–40.
Gosden RG, Hunter RHF, Telfer E, Torrance C, Brown N. Physiological factors underlying the formation of ovarian follicular fluid. Reproduction. 1988;82:813–25.
Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Zoology. 1967;58(2):560–7.
Clark AR, Stokes YM, Lane M, Thompson JG. Mathematical modelling of oxygen concentration in bovine and murine cumulus–oocyte complexes. Reproduction. 2006;131:999–1006.
Dan-Goor M, Sasson S, Davarashvili A, Almagor M. Expression of glucose transporter and glucose uptake in human oocytes and preimplantation embryos. Hum Reprod. 1997;12:2508–10.
von Mengden L, Klamt F, Smitz J. Redox biology of human cumulus cells: basic concepts, impact on oocyte quality, and potential clinical use. Antioxid Redox Signal. 2020;32:522–35.
Sanchez F, Adriaenssens T, Romero S, Smitz J. Quantification of oocyte-specific transcripts in follicle-enclosed oocytes during antral development and maturation in vitro. Mol Hum Reprod. 2009;15:539–50.
Akin N, von Mengden L, Herta A-C, Billooye K, van Leersum J, Cava-Cami B, et al. Glucose metabolism characterization during mouse in vitro maturation identifies alterations in cumulus cells†. Biol Reprod. 2021;104:902–13.
Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–8.
Wycherley G, Kane MT, Hynes AC. Oxidative phosphorylation and the tricarboxylic acid cycle are essential for normal development of mouse ovarian follicles. Hum Reprod. 2005;20:2757–63.
Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48:798–806.
Stine ZE, Altman BJ, Hsieh AL, Gouw AM, Dang CV. Deregulation of the cellular energetics of cancer cells. Pathobiology of Human Disease [Internet]. Elsevier; 2014 [cited 2022 Oct 20]. [444–55 p.]. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780123864567019122
Berg JM, Tymoczko JL, Gatto GJ Jr. Stryer L. 20.3 The pentose phosphate pathway generates NADPH and synthesizes five-carbon sugars. Biochemistry. 8th ed. New York: W. H. Freeman and Company; 2015. p. 601–7.
Sanchez-Lazo L, Brisard D, Elis S, Maillard V, Uzbekov R, Labas V, et al. Fatty acid synthesis and oxidation in cumulus cells support oocyte maturation in bovine. Mol Endocrinol. 2014;28:1502–21.
Lushchak OV, Piroddi M, Galli F, Lushchak VI. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep. 2014;19:8–15.
Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life; 2012. p. 362–9.
Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159–77.
Akin N, Ates G, Mengden L von, Herta AC, Meriggioli C, Billooye K, et al. Effects of lactate, super-GDF9 and low oxygen tension during biphasic in vitro maturation on the bioenergetic profiles of mouse cumulus-oocyte-complex. bioRxiv; 2022. https://doi.org/10.1101/2022.11.09.514870
Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101.
Feng Y, Cui P, Lu X, Hsueh B, Möller Billig F, Zarnescu Yanez L, et al. CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions. Sci Rep. 2017;7:44810.
Boland NI, Humpherson PG, Leese HJ, Gosden RG. Characterization of follicular energy metabolism. Hum Reprod. 1994;9:604–9.
Redding G, Bronlund J, Hart A. Theoretical investigation into the dissolved oxygen levels in follicular fluid of the developing human follicle using mathematical modeling. Reprod Fertil Dev. 2008;20:408–17.
Gook DA, Edgar DH, Lewis K, Sheedy JR, Gardner DK. Impact of oxygen concentration on adult murine pre-antral follicle development in vitro and the corresponding metabolic profile. Mol Hum Reprod. 2014;20(1):31–41.
Banwell KM, Lane M, Russell DL, Kind KL, Thompson JG. Oxygen concentration during mouse oocyte in vitro maturation affects embryo and fetal development. Hum Reprod. 2007;22:2768–75.
Makanji Y, Tagler D, Pahnke J, Shea LD, Woodruff TK. Hypoxia-mediated carbohydrate metabolism and transport promote early-stage murine follicle growth and survival. Physiol Endocrinol Metab. 2014;306(8):893–903.
Funding
This study was funded by Flanders FWO The Excellence of Science (EOS, FWO- F.R.S.-FNRS; G0F3118N) and by the Brazilian funds MCTI (Ministério da Ciência, Tecnologia e Inovações)/CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnologico) INCTTM (Instituto Nacional de Ciência e Tecnologia Translacional em Medicina)/CAPES (Coordenaçao de Aperfeiçoamento de Pessoal de Nivel Superior)/FAPESP (465458/2014-9) and PDSE-CAPES (Programa de Doutorado Sanduiche no Exterior-Coordenaçao de Aperfeiçoamento de Pessoal de Nivel Superior) 47/2017 (88881.188914/2018-01).
Author information
Authors and Affiliations
Contributions
ACH, LVM, NA: designed the experiments, performed the cultures, collected the samples, analyzed and interpreted the data, and prepared the manuscript. WC: performed the statistical analysis for the enzymatic assays. KB: provided help in the cultures and sample collection. BCC: provided technical assistance for enzyme assays. LSC: provided supervision on the data analysis and revised the manuscript. FK, JS, EA: supervised the project and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Supplementary fig. 1 Redox metabolism (A – D) and antioxidant capacity (E – F) were measured in in vitro grown mouse antral follicles via enzymatic assays. Samples from individual cell types (oocytes, GCs, CCs) were collected at GV stage, before the meiotic trigger and 18 hours after hCG/EGF stimulation from meiotically blocked (MB) and successfully matured (MII) antral follicles. Results were obtained from pools of 5 oocytes and corresponding somatic cells, from 3 independent experiments. For each sample type, from all 3 conditions, six biological replicates were measured as single technical replicates. ANOVA with Sidak’s multiple comparison test was performed to detect statistical significance (p<0.05) between the relevant comparisons. (DOCX 62.2 kb)
ESM 2
Supplementary Fig. 2. Glucose and redox metabolism in in vitro grown mouse antral follicles before (A) and after meiotic stimulus (B, C). All 3 cell types perform glycolysis, TCA cycle, PPP, OXPHOS and redox activities at different rates in immature (GV) as well as in both successfully matured (MII) and meiotically blocked (MB) follicles. Granulosa cells (GCs) play a major role in the follicle’s metabolic support prior (A) and after meiotic trigger (B, C). As the main site for lactic acid fermentation (purple circles) and glycolysis (pink circles), after the meiotic trigger, GCs share comparable levels of TCA cycle (orange circles) compared to the oocyte, while the latter is in charge of PPP. Cumulus cells (CCs) display significantly higher PPP and TCA cycle activities with lower malate levels following blocked maturation. No differences were detected in blocked oocytes for any of the metabolic markers after GV and MII stages comparisons. Similar metabolic trends were depicted after blocked (C) and successful (B) meiotic resumption with no significant differences detected between the two. LAF: Lactic Acid Fermentation; OXPHOS: Oxidative Phosphorylation. PPP: Pentose Phosphate Pathway; NADP+: nicotinamide adenine dinucleotide phosphate; SOD: Superoxide Dismutase; GST: Glutathione-S-Transferase; TAC: Total Antioxidant Capacity; SMAC: Small Molecules Antioxidant Capacity. The follicle compartments are color coded (as displayed in 8A): light orange for GCs, purple for CCs and light blue for the oocyte. A and B were adapted from Herta et al. 2022 following the permission by Oxford University press (license number: 5553701022789, obtained on May 21, 2023). (DOCX 9.35 MB)
ESM 3
Supplementary Table 1. Enzymatic assays kits used in this study. (DOCX 29.6 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herta, AC., von Mengden, L., Akin, N. et al. Glucose and redox metabolism in meiotically blocked in vitro grown mouse antral follicles. J Assist Reprod Genet 40, 2851–2863 (2023). https://doi.org/10.1007/s10815-023-02940-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02940-7