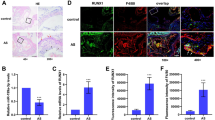
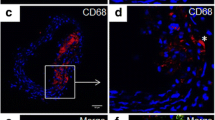
Abstract
Macrophage-derived lipid-laden foam cells from the subendothelium play a crucial role in the initiation and progression of atherosclerosis. However, the molecule mechanism that regulates the formation of foam cells is not completely understood. Here, we found that SLAMF7 was upregulated in mice bone marrow–derived macrophages and RAW264.7 cells stimulated with oxidized low-density lipoprotein (ox-LDL). SLAMF7 promoted ox-LDL-mediated macrophage lipid accumulation and M1-type polarization. SLAMF7 deficiency reduced serum lipid levels and improved the lesions area of carotid plaque and aortic arch in high-fat diet-fed ApoE−/− mice. In response to ox-LDL, SLAMF7 downregulated NR4A1 and upregulated RUNX3 through transcriptome sequencing analysis. Overexpression NR4A1 reversed SLAMF7-induced lipid uptake and M1 polarization via inhibiting RUNX3 expression. Furthermore, RUNX3 enhanced foam cell formation and M1-type polarization. Taken together, the study suggested that SLAMF7 play contributing roles in the pro-atherogenic effects by regulating NR4A1-RUNX3.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bonati L. H., O. Jansen, G. J. de Borst, M. M. Brown. 2022. Management of atherosclerotic extracranial carotid artery stenosis. Lancet Neurology 21 (3):273–283. https://doi.org/10.1016/S1474-4422(21)00359-8.
Tzoulaki I., R. Castagné, C. L. Boulangé, I. Karaman, E. Chekmeneva, E. Evangelou, T. M. D. Ebbels, M. R. Kaluarachchi, M. Chadeau-Hyam, D. Mosen, A. Dehghan, A. Moayyeri, D. L. S. Ferreira, X. Guo, J. I. Rotter, K. D. Taylor, M. Kavousi, P. S. de Vries, B. Lehne, M. Loh, A. Hofman, J. K. Nicholson, J. Chambers, C. Gieger, E. Holmes, R. Tracy, J. Kooner, P. Greenland, O. H. Franco, D. Herrington, J. C. Lindon, P. Elliott. 2019. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. European Heart Journal 40 (34):2883–2896. https://doi.org/10.1093/eurheartj/ehz235.
Liu Z., H. Zhu, X. Dai, C. Wang, Y. Ding, P. Song, M. H. Zou. 2017. Macrophage liver kinase B1 inhibits foam cell formation and atherosclerosis. Circulation Research 121 (9):1047–1057. https://doi.org/10.1161/CIRCRESAHA.117.311546.
Libby P., J. E. Buring, L. Badimon, G. K. Hansson, J. Deanfield, M. S. Bittencourt, L. Tokgozoglu, E. F. Lewis. 2019. Atherosclerosis. Nature Reviews Disease Primers 5 (1):56. https://doi.org/10.1038/s41572-019-0106-z.
Summerhill V. I., A. V. Grechko, S. F. Yet, I. A. Sobenin, A. N. Orekhov. 2019. The atherogenic role of circulating modified lipids in atherosclerosis. International Journal of Molecular Sciences 20 (14). https://doi.org/10.3390/ijms20143561.
Hartley A., D. Haskard, R. Khamis. 2019. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis - novel insights and future directions in diagnosis and therapy<sup/>. Trends in Cardiovascular Medicine 29 (1):22–26. https://doi.org/10.1016/j.tcm.2018.05.010.
Tabas I., K. E. Bornfeldt. 2020. Intracellular and intercellular aspects of macrophage immunometabolism in atherosclerosis. Circulation Research 126 (9):1209–1227. https://doi.org/10.1161/CIRCRESAHA.119.315939.
Back M., A. Yurdagul, Jr., I. Tabas, K. Oorni, P. T. Kovanen. 2019. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nature Reviews Cardiology 16 (7):389–406. https://doi.org/10.1038/s41569-019-0169-2.
Libby P., G. Pasterkamp, F. Crea, I. K. Jang. 2019. Reassessing the mechanisms of acute coronary syndromes. Circulation Research 124 (1):150–160. https://doi.org/10.1161/CIRCRESAHA.118.311098.
Doddapattar P., R. Dev, M. Ghatge, R. B. Patel, M. Jain, N. Dhanesha, S. R. Lentz, A. K. Chauhan. 2022. Myeloid cell PKM2 deletion enhances efferocytosis and reduces atherosclerosis. Circulation Research 130 (9):1289–1305. https://doi.org/10.1161/CIRCRESAHA.121.320704.
Malaer J. D., A. M. Marrufo, P. A. Mathew. 2019. 2B4 (CD244, SLAMF4) and CS1 (CD319, SLAMF7) in systemic lupus erythematosus and cancer. Clinical Immunology 204:50–56. https://doi.org/10.1016/j.clim.2018.10.009.
Cannons J. L., S. G. Tangye, P. L. Schwartzberg. 2011. SLAM family receptors and SAP adaptors in immunity. Annual Review of Immunology 29:665–705. https://doi.org/10.1146/annurev-immunol-030409-101302.
Lonial S., M. Dimopoulos, A. Palumbo, D. White, S. Grosicki, I. Spicka, A. Walter-Croneck, P. Moreau, M. V. Mateos, H. Magen, A. Belch, D. Reece, M. Beksac, A. Spencer, H. Oakervee, R. Z. Orlowski, M. Taniwaki, C. Rollig, H. Einsele, K. L. Wu, A. Singhal, J. San-Miguel, M. Matsumoto, J. Katz, E. Bleickardt, V. Poulart, K. C. Anderson, P. Richardson, E.-. Investigators. 2015. Elotuzumab therapy for relapsed or refractory multiple myeloma. The New England Journal of Medicine 373 (7):621–631. https://doi.org/10.1056/NEJMoa1505654.
O’Neal J., J. K. Ritchey, M. L. Cooper, J. Niswonger, L. Sofia Gonzalez, E. Street, M. P. Rettig, S. W. Gladney, L. Gehrs, R. Abboud, J. L. Prior, G. J. Haas, R. G. Jayasinghe, L. Ding, A. Ghobadi, R. Vij, J. F. DiPersio. 2022. CS1 CAR-T targeting the distal domain of CS1 (SLAMF7) shows efficacy in high tumor burden myeloma model despite fratricide of CD8+CS1 expressing CAR-T cells. Leukemia 36 (6):1625–1634. https://doi.org/10.1038/s41375-022-01559-4.
O’Connell P., M. K. Blake, S. Godbehere, A. Amalfitano, Y. A. Aldhamen. 2022. SLAMF7 modulates B cells and adaptive immunity to regulate susceptibility to CNS autoimmunity. Journal of Neuroinflammation 19 (1):241. https://doi.org/10.1186/s12974-022-02594-9.
Comte D., M. P. Karampetsou, N. Yoshida, K. Kis-Toth, V. C. Kyttaris, G. C. Tsokos. 2017. Signaling lymphocytic activation molecule family member 7 engagement restores defective effector CD8+ T cell function in systemic lupus erythematosus. Arthritis Rheumatology 69 (5):1035–1044. https://doi.org/10.1002/art.40038.
O’Connell P., Y. Pepelyayeva, M. K. Blake, S. Hyslop, R. B. Crawford, M. D. Rizzo, C. Pereira-Hicks, S. Godbehere, L. Dale, P. Gulick, N. E. Kaminski, A. Amalfitano, Y. A. Aldhamen. 2019. SLAMF7 is a critical negative regulator of IFN-alpha-mediated CXCL10 production in chronic HIV infection. The Journal of Immunology 202 (1):228–238. https://doi.org/10.4049/jimmunol.1800847.
Wu Y., Q. Wang, M. Li, J. Lao, H. Tang, S. Ming, M. Wu, S. Gong, L. Li, L. Liu, X. Huang. 2023. SLAMF7 regulates the inflammatory response in macrophages during polymicrobial sepsis. The Journal of Clinical Investigation 133 (6). https://doi.org/10.1172/jci150224.
Zhu S., Y. Chen, J. Lao, C. Wu, X. Zhan, Y. Wu, Y. Shang, Z. Zou, J. Zhou, X. Ji, X. Huang, X. Shi, M. Wu. 2021. Signaling lymphocytic activation molecule family-7 alleviates corneal inflammation by promoting M2 polarization. The Journal of Infectious Diseases 223 (5):854–865. https://doi.org/10.1093/infdis/jiaa445.
Simmons D. P., H. N. Nguyen, E. Gomez-Rivas, Y. Jeong, A. H. Jonsson, A. F. Chen, J. K. Lange, G. S. Dyer, P. Blazar, B. E. Earp, J. S. Coblyn, E. M. Massarotti, J. A. Sparks, D. J. Todd, R. A. S. L. E. N. Accelerating Medicines Partnership, D. A. Rao, E. Y. Kim, M. B. Brenner. 2022. SLAMF7 engagement superactivates macrophages in acute and chronic inflammation. Science Immunology 7 (68):eabf2846. https://doi.org/10.1126/sciimmunol.abf2846.
Xia, Z.G.M., X. Jia, X. Wang, C. Wu, J. Guo, L. Zhang, Y. Du, and J. Wang. 2018. Integrated DNA methylation and gene expression analysis identifies SLAMF7 as a key regulator of atherosclerosis. Aging 10 (6): 1324–1337.
Wang C., W. Yang, X. Liang, W. Song, J. Lin, Y. Sun, X. Guan. 2020. MicroRNA-761 modulates foam cell formation and inflammation through autophagy in the progression of atherosclerosis. Molecular and Cellular Biochemistry 474 (1–2):135–146. https://doi.org/10.1007/s11010-020-03839-y.
Fang S., X. Wan, X. Zou, S. Sun, X. Hao, C. Liang, Z. Zhang, F. Zhang, B. Sun, H. Li, B. Yu. 2021. Arsenic trioxide induces macrophage autophagy and atheroprotection by regulating ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell Death & Disease 12 (1):88. https://doi.org/10.1038/s41419-020-03357-1.
Fu X., X. Yu, J. Jiang, J. Yang, L. Chen, Z. Yang, C. Yu. 2022. Small molecule-assisted assembly of multifunctional ceria nanozymes for synergistic treatment of atherosclerosis. Nature Communications 13 (1):6528. https://doi.org/10.1038/s41467-022-34248-y.
Yang Z., Y. Huang, L. Zhu, K. Yang, K. Liang, J. Tan, B. Yu. 2021. SIRT6 promotes angiogenesis and hemorrhage of carotid plaque via regulating HIF-1alpha and reactive oxygen species. Cell Death & Disease 12 (1):77. https://doi.org/10.1038/s41419-020-03372-2.
Zhang K., J. Zheng, Y. Chen, J. Dong, Z. Li, Y. P. Chiang, M. He, Q. Huang, H. Tang, X. C. Jiang. 2021. Inducible phospholipid transfer protein deficiency ameliorates atherosclerosis. Atherosclerosis 324:9–17. https://doi.org/10.1016/j.atherosclerosis.2021.03.011.
Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. Babraham Bioinformatics, Babraham Institute, Cambridge, United Kingdom,
Liao Y., G. K. Smyth, W. Shi. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics (Oxford, England) 30 (7):923–930. https://doi.org/10.1093/bioinformatics/btt656.
Love M. I., W. Huber, S. Anders. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15 (12):550. https://doi.org/10.1186/s13059-014-0550-8.
Benjamini Y., Y. J. J. o. t. R. s. s. s. B. Hochberg. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. 57 (1):289–300.
Johnston JM A. A., Bauer RC, Hamby S, Suvarna SK, Baidžajevas K, Hegedus Z, Dear TN, Turner M; Cardiogenics Consortium; Wilson HL, Goodall AH, Rader DJ, Shoulders CC, Francis SE, Kiss-Toth E. 2019. Myeloid Tribbles 1 induces early atherosclerosis via enhanced foam cell expansion. Science Advances 5 (10):eaax9183.
Li H., Z. Cao, L. Wang, C. Liu, H. Lin, Y. Tang, P. Yao. 2022. Macrophage subsets and death are responsible for atherosclerotic plaque formation. Front Immunology 13:843712. https://doi.org/10.3389/fimmu.2022.843712.
Schwarz N., S. Fernando, Y. C. Chen, T. Salagaras, S. R. Rao, S. Liyanage, A. E. Williamson, D. Toledo-Flores, C. Dimasi, T. J. Sargeant, J. Manavis, E. Eddy, P. Kanellakis, P. L. Thompson, J. T. M. Tan, M. F. Snel, C. A. Bursill, S. J. Nicholls, K. Peter, P. J. Psaltis. 2023. Colchicine exerts anti-atherosclerotic and -plaque-stabilizing effects targeting foam cell formation. FASEB Journal 37 (4):e22846. https://doi.org/10.1096/fj.202201469R.
Hanna R. N., I. Shaked, H. G. Hubbeling, J. A. Punt, R. Wu, E. Herrley, C. Zaugg, H. Pei, F. Geissmann, K. Ley, C. C. Hedrick. 2012. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circulation Research 110 (3):416–427. https://doi.org/10.1161/CIRCRESAHA.111.253377.
Hamers A. A., M. Vos, F. Rassam, G. Marinkovic, K. Kurakula, P. J. van Gorp, M. P. de Winther, M. J. Gijbels, V. de Waard, C. J. de Vries. 2012. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circulation Research 110 (3):428–438. https://doi.org/10.1161/CIRCRESAHA.111.260760.
Koenis D. S., L. Medzikovic, P. B. van Loenen, M. van Weeghel, S. Huveneers, M. Vos, I. J. Evers-van Gogh, J. Van den Bossche, D. Speijer, Y. Kim, L. Wessels, N. Zelcer, W. Zwart, E. Kalkhoven, C. J. de Vries. 2018. Nuclear receptor Nur77 limits the macrophage inflammatory response through transcriptional reprogramming of mitochondrial metabolism. Cell Reports 24 (8):2127–2140 e2127. https://doi.org/10.1016/j.celrep.2018.07.065.
Nowyhed H. N., T. R. Huynh, A. Blatchley, R. Wu, G. D. Thomas, C. C. Hedrick. 2015. The nuclear receptor nr4a1 controls CD8 T cell development through transcriptional suppression of runx3. Scientific Reports 5:9059. https://doi.org/10.1038/srep09059.
Song P., Z. Fang, H. Wang, Y. Cai, K. Rahimi, Y. Zhu, F. G. R. Fowkes, F. J. I. Fowkes, I. Rudan. 2020. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Global Health 8 (5):e721-e729. https://doi.org/10.1016/S2214-109X(20)30117-0.
Lin H. P., B. Singla, W. Ahn, P. Ghoshal, M. Blahove, M. Cherian-Shaw, A. Chen, A. Haller, D. Y. Hui, K. Dong, J. Zhou, J. White, A. M. Stranahan, A. Jasztal, R. Lucas, B. K. Stansfield, D. Fulton, S. Chlopicki, G. Csanyi. 2022. Receptor-independent fluid-phase macropinocytosis promotes arterial foam cell formation and atherosclerosis. Science Translational Medicine 14 (663):eadd2376. https://doi.org/10.1126/scitranslmed.add2376.
Liu X., J. W. Guo, X. C. Lin, Y. H. Tuo, W. L. Peng, S. Y. He, Z. Q. Li, Y. C. Ye, J. Yu, F. R. Zhang, M. M. Ma, J. Y. Shang, X. F. Lv, A. D. Zhou, Y. Ouyang, C. Wang, R. P. Pang, J. X. Sun, J. S. Ou, J. G. Zhou, S. J. Liang. 2021. Macrophage NFATc3 prevents foam cell formation and atherosclerosis: evidence and mechanisms. European Heart Journal 42 (47):4847–4861. https://doi.org/10.1093/eurheartj/ehab660.
Wang D., Y. Yang, Y. Lei, N. T. Tzvetkov, X. Liu, A. W. K. Yeung, S. Xu, A. G. Atanasov. 2019. Targeting foam cell formation in atherosclerosis: therapeutic potential of natural products. Pharmacological Reviews 71 (4):596–670. https://doi.org/10.1124/pr.118.017178.
Wang B., X. Tang, L. Yao, Y. Wang, Z. Chen, M. Li, N. Wu, D. Wu, X. Dai, H. Jiang, D. Ai. 2022. Disruption of USP9X in macrophages promotes foam cell formation and atherosclerosis. Journal of Clinical Investigation 132 (10). https://doi.org/10.1172/JCI154217.
Pi S., L. Mao, J. Chen, H. Shi, Y. Liu, X. Guo, Y. Li, L. Zhou, H. He, C. Yu, J. Liu, Y. Dang, Y. Xia, Q. He, H. Jin, Y. Li, Y. Hu, Y. Miao, Z. Yue, B. Hu. 2021. The P2RY12 receptor promotes VSMC-derived foam cell formation by inhibiting autophagy in advanced atherosclerosis. Autophagy 17 (4):980–1000. https://doi.org/10.1080/15548627.2020.1741202.
Spann N. J., L. X. Garmire, J. G. McDonald, D. S. Myers, S. B. Milne, N. Shibata, D. Reichart, J. N. Fox, I. Shaked, D. Heudobler, C. R. Raetz, E. W. Wang, S. L. Kelly, M. C. Sullards, R. C. Murphy, A. H. Merrill, Jr., H. A. Brown, E. A. Dennis, A. C. Li, K. Ley, S. Tsimikas, E. Fahy, S. Subramaniam, O. Quehenberger, D. W. Russell, C. K. Glass. 2012. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151 (1):138–152. https://doi.org/10.1016/j.cell.2012.06.054.
Engelen S. E., A. J. B. Robinson, Y. X. Zurke, C. Monaco. 2022. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nature Reviews Cardiology 19 (8):522–542. https://doi.org/10.1038/s41569-021-00668-4.
Jinnouchi H., L. Guo, A. Sakamoto, S. Torii, Y. Sato, A. Cornelissen, S. Kuntz, K. H. Paek, R. Fernandez, D. Fuller, N. Gadhoke, D. Surve, M. Romero, F. D. Kolodgie, R. Virmani, A. V. Finn. 2020. Diversity of macrophage phenotypes and responses in atherosclerosis. Cellular and Molecular Life Sciences 77 (10):1919–1932. https://doi.org/10.1007/s00018-019-03371-3.
Ruosen Y., W. Zhang, P. Nie, K. Lan, X. Yang, A. Yin, Q. Xiao, Y. Shen, K. Xu, X. Wang, X. Pan, L. Shen, B. He. 2022. Nur77 deficiency exacerbates macrophage NLRP3 inflammasome-mediated inflammation and accelerates atherosclerosis. Oxidative Medicine and Cellular Longevity 2022:2017815. https://doi.org/10.1155/2022/2017815.
Nus M., G. Basatemur, M. Galan, L. Cros-Brunso, T. X. Zhao, L. Masters, J. Harrison, N. Figg, D. Tsiantoulas, F. Geissmann, C. J. Binder, A. P. Sage, Z. Mallat. 2020. NR4A1 deletion in marginal zone B cells exacerbates atherosclerosis in mice-brief report. Arteriosclerosis, Thrombosis, and Vascular Biology 40 (11):2598–2604. https://doi.org/10.1161/ATVBAHA.120.314607.
Bao M. H., H. Q. Luo, L. H. Chen, L. Tang, K. F. Ma, J. Xiang, L. P. Dong, J. Zeng, G. Y. Li, J. M. Li. 2016. Impact of high fat diet on long non-coding RNAs and messenger RNAs expression in the aortas of ApoE(-/-) mice. Scientific Reports 6:34161. https://doi.org/10.1038/srep34161.
Su Z., H. Lu, H. Jiang, H. Zhu, Z. Li, P. Zhang, P. Ni, H. Shen, W. Xu, H. Xu. 2015. IFN-gamma-producing Th17 cells bias by HMGB1-T-bet/RUNX3 axis might contribute to progression of coronary artery atherosclerosis. Atherosclerosis 243 (2):421–428. https://doi.org/10.1016/j.atherosclerosis.2015.09.037.
Shao Y., F. Saaoud, W. Cornwell, K. Xu, A. Kirchhoff, Y. Lu, X. Jiang, H. Wang, T. J. Rogers, X. Yang. 2022. Cigarette smoke and morphine promote treg plasticity to Th17 via enhancing trained immunity. Cells 11 (18). https://doi.org/10.3390/cells11182810.
Dybska E., J. K. Nowak, J. Walkowiak. 2023. Transcriptomic context of RUNX3 expression in monocytes: a cross-sectional analysis. Biomedicines 11 (6). https://doi.org/10.3390/biomedicines11061698.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020MH139) and Shandong Province Postdoctoral Innovation Project (2021151).
Author information
Authors and Affiliations
Contributions
Fengjiao Yuan and Jianmei Wei designed study, performed research, analyzed data, and wrote the paper. Yan Cheng and Feifei Wang performed research. Mingliang Gu and Yanhui Li guided experimental techniques. Xin Zhao, Hao Song, and Ru Ban interpreted pathology. Jing Zhou and Zhangyong Xia designed study, and revised the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Animal experiment was conducted according to the Institutional Animal Care and Use Committee of the Model Animal Research Center. Approval was granted by the Ethics Committee of Liaocheng People’s Hospital.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, F., Wei, J., Cheng, Y. et al. SLAMF7 Promotes Foam Cell Formation of Macrophage by Suppressing NR4A1 Expression During Carotid Atherosclerosis. Inflammation 47, 530–542 (2024). https://doi.org/10.1007/s10753-023-01926-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01926-y