Abstract—
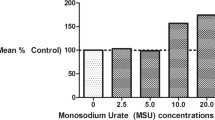
Polygallic acid (PGAL) has been used in vitro to protect synoviocytes from monosodium urate (MSU) crystals due to its anti-inflammatory properties. However, MSU crystals can also activate other cells of the synovial fluid (SF). We studied the impact of PGAL on the phagocytosis of MSU crystals, inflammation, and oxidative stress using an in vitro model with SF leukocytes and THP-1 monocyte cells. SF leukocytes were stimulated with PGAL and MSU crystals, proinflammatory cytokines and phagocytosis were assessed. In THP-1 cells, the effect of PGAL on the phagocytosis of MSU crystals and the levels of IL-1β, IL-6, TNF-α, and reactive oxygen species (ROS) was evaluated. PGAL was added to THP-1 cultures 24 h before MSU crystal addition as a pre-treatment, and IL-1β was measured. One-way ANOVA with Tukey's post hoc test was performed, and a P value < 0.05 was considered statistically significant. PGAL (100 µg/mL) decreased phagocytosis in SF leukocytes by 14% compared to cells exposed to crystals without PGAL. In THP-1 cells, 100 and 200 µg/mL PGAL reduced phagocytosis by 17% and 15%, respectively. In SF cells, there was a tendency to decrease IL-1β and IL-6. In THP-1 cells, decreases in IL-1β and TNF-α, as well as a slight decrease in ROS, were identified. PGAL pre-treatment resulted in a reduction of IL-1β. PGAL inhibits MSU phagocytosis by exerting an anti-inflammatory effect on cells exposed to crystals. The use of PGAL before an acute attack of gout suggests an important protective factor to control the inflammation.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Dehlin, M., L. Jacobsson, and E. Roddy. 2020. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nature Reviews Rheumatology 16: 380–390.
Wen, P., P. Luo, B. Zhang, and Y. Zhang. 2022. Mapping knowledge structure and global research trends in gout: a bibliometric analysis from 2001 to 2021. Frontiers in Public Health 29 (10): 924676.
Ahmad, M.I., S. Masood, D.M. Furlanetto, and S. Nicolaou. 2021. Urate crystals; beyond joints. Frontiers in Medicine 8: 649505.
Oda, M., I. Hirasawa, and F. Kohori. 2020. Analysis of morphological changes in monosodium urate monohydrate crystals for gout treatment. Chemical Engineering and Technology 43: 1087–1092.
Popov, D., L. Jain, M. Alhilali, N. Dalbeth, and R.C. Poulsen. 2023. Monosodium urate crystals alter the circadian clock in macrophages leading to loss of NLRP3 inflammasome repression: Implications for timing of the gout flare. The FASEB Journal 37: e22940.
Qadri, M., G.D. Jay, L.X. Zhang, W. Wong, A.M. Reginato, C. Sun, T.A. Schmidt, and K.A. Elsaid. 2018. Recombinant human proteoglycan-4 reduces phagocytosis of urate crystals and downstream nuclear factor kappa B and inflammasome activation and production of cytokines and chemokines in human and murine macrophages. Arthritis Research & Therapy 20: 192.
Bousoik, E., M. Qadri, and K.A. Elsaid. 2020. CD44 receptor mediates urate crystal phagocytosis by macrophages and regulates inflammation in a murine peritoneal model of acute gout. Science and Reports 10: 5748.
Fernandes, M.J., and P.H. Naccache. 2018. The role of inhibitory receptors in monosodium urate crystal-induced inflammation. Frontiers in Immunology 9: 1883.
Vedder, D., M. Gerritsen, B. Duvvuri, R.F. van Vollenhoven, M.T. Nurmohamed, and C. Lood. 2020. Neutrophil activation identifies patients with active polyarticular gout. Arthritis Research & Therapy 22: 148.
Davidsson, L., A. Dahlstrand Rudin, F.P. Sanchez Klose, A. Buck, L. Björkman, K. Christenson, and J. Bylund. 2020. In vivo transmigrated human neutrophils are highly primed for intracellular radical production induced by monosodium urate crystals. International Journal of Molecular Sciences 21: 3750.
Desai, J., S. Steiger, and H.J. Anders. 2017. Molecular pathophysiology of gout. Trends in Molecular Medicine 23: 756–768.
Balik, A.R., A. Omma, S.C. Sandikci, C. Yucel, M. Kizilgun, Z.B. Balik, E.F. Oguz, S. Neselioglu, and O. Erel. 2022. Evaluation of oxidative stress in gout disease; thiol-disulfide homeostasis and ischemia-modified albumin levels. International Journal of Medical Biochemistry 5: 109–115.
Cabău, G., T.O. Crișan, V. Klück, R.A. Popp, and L.A.B. Joosten. 2020. Urate-induced immune programming: consequences for gouty arthritis and hyperuricemia. Immunological Reviews 294: 92–105.
Pan, Y.G., M.T. Huang, P. Sekar, D.Y. Huang, W.W. Lin, and S.L. Hsieh. 2021. Decoy receptor 3 inhibits monosodium urate-induced NLRP3 inflammasome activation via reduction of reactive oxygen species production and lysosomal rupture. Frontiers in Immunology 3 (12): 638676.
Scanu, A., R. Luisetto, R. Ramonda, P. Spinella, P. Sfriso, P. Galozzi, and F. Oliviero. 2022. Anti-inflammatory and hypouricemic effect of bioactive compounds: molecular evidence and potential application in the management of gout. Current Issues in Molecular Biology 44: 5173–5190.
Zamudio-Cuevas, Y., M.A. Andonegui-Elguera, A. Aparicio-Juárez, E. Aguillón-Solís, K. Martínez-Flores, E. Ruvalcaba-Paredes, et al. 2021. The enzymatic poly(gallic acid) reduces pro-inflammatory cytokines in vitro, a potential application in inflammatory diseases. Inflammation 44: 174–185.
Zamudio-Cuevas, Y., V. Martínez-López, I.A. Luján-Juárez, N. Montaño-Armendariz, K. Martínez-Flores, J. Fernández-Torres, M. Gimeno, and R. Sánchez-Sánchez. 2022. Anti-inflammatory and antioxidant effect of poly-gallic acid (PGAL) in an in vitro model of synovitis induced by monosodium urate crystals. Inflammation 45: 2066–2077.
López, J., J.M. Hernández-Alcántara, P. Roquero, C. Montiel, K. Shirai, M. Gimeno, and E. Bárzana. 2013. Trametes versicolor laccase oxidation of gallic acid toward apolyconjugated semiconducting material. Journal of Molecular Catalysis B: Enzymatic 97: 100–105.
Vona, D., G. Buscemi, R. Ragni, M. Cantore, S. Cicco, G. Farinola, and M. Trotta. 2020. Synthesis of (poly)gallic acid in a bacterial growth medium. MRS Advances 5: 957–963.
Romero-Montero, A., M. Gimeno, N. Farfán, and P. Labra-Vázquez. 2019. Enzymatic poly (gallic acid): a stable multiradical polyanion. Journal of Molecular Structure 1197: 326–335.
Scanu, A., F. Oliviero, L. Gruaz, P. Galozzi, R. Luisetto, R. Ramonda, D. Burger, and L. Punzi. 2016. Synovial fluid proteins are required for the induction of interleukin-1β production by monosodium urate crystals. Scandinavian Journal of Rheumatology 45: 384–393.
Jeong, J.H., J.H. Jung, J.S. Lee, J.S. Oh, Y.G. Kim, C.K. Lee, B. Yoo, and S. Hong. 2019. Prominent inflammatory features of monocytes/macrophages in acute calcium pyrophosphate crystal arthritis: a comparison with acute gouty arthritis. Immune Network 19: e21.
Zamudio-Cuevas, Y., G.A. Martínez-Nava, K. Martínez-Flores, L. Ventura-Ríos, J. Vazquez-Mellado, P. Rodríguez-Henríquez, C. Pineda, R. Franco-Cendejas, C.A. Lozada-Pérez, and J. Fernández-Torres. 2021. Synovial fluid analysis for the enhanced clinical diagnosis of crystal arthropathies in a tertiary care institution. Clinical Rheumatology 40: 3239–3246.
Oliviero, F., Y. Zamudio-Cuevas, E. Belluzzi, L. Andretto, A. Scanu, M. Favero, R. Ramonda, G. Ravagnan, A. López-Reyes, P. Spinella, and L. Punzi. 2019. Polydatin and resveratrol inhibit the inflammatory process induced by urate and pyrophosphate crystals in THP-1 cells. Foods 8: 560.
Huang, Q., Y. Huang, X. Guo, J. Chen, Z. Zhong, Y. Liu, W. Deng, and T. Li. 2021. The diagnostic value of synovial fluid lymphocytes in gout patients. Disease Markers 2021: 4385611.
Kundu, S., A. Bala, P. Ghosh, D. Mukhopadhyay, A. Mitra, A. Sarkar, A.K. Bauri, A. Ghosh, S. Chattopadhyay, and M. Chatterjee. 2011. Attenuation of oxidative stress by allylpyrocatechol in synovial cellular infiltrate of patients with Rheumatoid Arthritis. Free Radical Research 45: 518–526.
Hsueh, M.F., M.P. Bolognesi, S.S. Wellman, and V.B. Kraus. 2020. Anti-inflammatory effects of naproxen sodium on human osteoarthritis synovial fluid immune cells. Osteoarthritis and Cartilage 28: 639–645.
Zhang, X., L. Hu, S. Xu, C. Ye, and A. Chen. 2021. Erianin: a direct NLRP3 inhibitor with remarkable anti-inflammatory activity. Frontiers in Immunology 12: 739953.
Tsuzuki, N., Y. Kanbayashi, and K. Kusano. 2019. Markers for oxidative stress in the synovial fluid of thoroughbred horses with carpal bone fracture. Journal of Equine Science 30: 13–16.
Pascual, E., and V. Jovaní. 1995. A quantitative study of the phagocytosis of urate crystals in the synovial fluid of asymptomatic joints of patients with gout. British Journal of Rheumatology 34: 724–726.
Baggio, C., P. Sfriso, A. Cignarella, P. Galozzi, A. Scanu, F. Mastrotto, M. Favero, R. Ramonda, and F. Oliviero. 2021. Phagocytosis and inflammation in crystal-induced arthritis: a synovial fluid and in vitro study. Clinical and Experimental Rheumatology 39: 494–500.
Yagnik, D.R., P. Hillyer, D. Marshall, C.D. Smythe, T. Krausz, D.O. Haskard, and R.C. Landis. 2000. Noninflammatory phagocytosis of monosodium urate monohydrate crystals by mouse macrophages. Implications for the control of joint inflammation in gout. Arthritis and Rheumatism 43: 1779–1789.
Dalbeth, N., and D.O. Haskard. 2005. Mechanisms of inflammation in gout. Rheumatology 44: 1090–1096.
Landis, R.C., D.R. Yagnik, O. Florey, P. Philippidis, V. Emons, J.C. Mason, and D.O. Haskard. 2002. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis and Rheumatism 46: 3026–3033.
Jeong, J.H., S.J. Choi, S.M. Ahn, J.S. Oh, Y.G. Kim, C.K. Lee, B. Yoo, and S. Hong. 2021. Neutrophil extracellular trap clearance by synovial macrophages in gout. Arthritis Research & Therapy 23: 88.
Liu, L., L. Zhu, M. Liu, L. Zhao, Y. Yu, Y. Xue, and L. Shan. 2022. Recent insights into the role of macrophages in acute gout. Frontiers in Immunology 13: 955806.
Vírgen Gen, J.J., R.I. Guzmán-Gerónimo, K. Martínez-Flores, G.A. Martínez-Nava, J. Fernández-Torres, and Y. Zamudio-Cuevas. 2020. Cherry extracts attenuate inflammation and oxidative stress triggered by monosodium urate crystals in THP-1 cells. Journal of Food Biochemistry 44: e13403.
Riaz, M., L.T. Al Kury, N. Atzaz, A. Alattar, R. Alshaman, F.A. Shah, and S. Li. 2022. Carvacrol alleviates hyperuricemia-induced oxidative stress and inflammation by modulating the NLRP3/NF-κB pathwayt. Drug Design, Development and Therapy 16: 1159–1170.
Furger, C. 2021. Live cell assays for the assessment of antioxidant activities of plant extracts. Antioxidants 10: 944.
Murphy, M.P., H. Bayir, V. Belousov, C.J. Chang, K.J.A. Davies, M.J. Davies, T.P. Dick, T. Finkel, et al. 2022. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nature Metabolism 4: 651–662.
Romero-Montero, A., L.J. Del Valle, J. Puiggalí, C. Montiel, R. García-Arrazola, and M. Gimeno. 2020. Poly(gallic acid)-coated polycaprolactone inhibits oxidative stress in epithelial cells. Materials Science & Engineering, C: Materials for Biological Applications 115: 111154.
Sánchez-Sánchez, R., A. Romero-Montero, C. Montiel, Y. Melgarejo-Ramírez, C. Sánchez-Ortega, H. Lugo-Martínez, B. Cabello-Arista, R. García-Arrazola, C. Velasquillo, and M. Gimeno. 2017. Cytoprotective effect of the enzyme-mediated polygallic acid on fibroblast cells under exposure of UV-irradiation. Materials Science & Engineering, C: Materials for Biological Applications 76: 417–424.
Renaudin, F., S. Sarda, L. Campillo-Gimenez, C. Séverac, T. Léger, C. Charvillat, C. Rey, F. Lioté, J.-M. Camadro, H.-K. Ea, and C. Combes. 2019. Adsorption of proteins on m-CPPD and urate crystals inhibits crystal-induced cell responses: study on albumin-crystal interaction. Journal of Functional Biomaterials 10: 18.
Scanu, A., R. Luisetto, F. Oliviero, L. Gruaz, P. Sfriso, D. Burger, and L. Punzi. 2015. High-density lipoproteins inhibit urate crystal-induced inflammation in mice. Annals of the Rheumatic Diseases 74: 587–594.
Acknowledgements
We would like to thank Jorge Cerna Cortés, Ph.D., from Instituto Politécnico Nacional (Mexico) for the reagents for carrying out cell culture assays and ELISAs.
Funding
This research received funding from the National Council of Science and Technology (CONACYT) for financing SEP-BASICA FSSEP02-C-2018-2 # A1-S-16109.
Author information
Authors and Affiliations
Contributions
YZC and JFT contributed to the conception and design of the study, data collection, analysis, and interpretation, and writing and critical revision of the manuscript. VML and ALM, cell cultures, oxidative stress experiments, and data acquisition. NMA and CALP, provision of SF samples, collection of medical records and critical revision of the manuscript. KMF and CHV, pro-inflammatory molecules quantification and acquisition of data, writing and critical revision of the manuscript. YZC and RSS, statistical analysis, data interpretation and critical revision of the manuscript. MG, data interpretation and critical revision of the manuscript. All authors contributed to data analysis and interpretation as well as writing and critical revision of the article. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Ethics and Research Committee of the Instituto Nacional de Rehabilitación Luis Guillermo Ibarra Ibarra (registration number INR-27/20). All procedures performed in this study involving human participants were in accordance with the ethical standards of the INR-LGII-Institutional Research and Ethical Committee and with the Helsinki Declaration (1964). Informed consent was obtained from all individual participants included in the study.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zamudio-Cuevas, Y., Martínez-López, V., López-Macay, A. et al. Antiphagocytic Properties of Polygallic Acid with Implications in Gouty Inflammation. Inflammation 46, 1952–1965 (2023). https://doi.org/10.1007/s10753-023-01852-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01852-z