Abstract
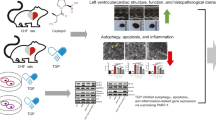
Although it is known that caffeic acid phenethyl ester (CAPE) and its derivatives could ameliorate acute myocardial injury, their effects on chronic myocardial ischemia (CMI) were not reported. This study aimed to investigate the potential effect of caffeic acid p-nitro phenethyl ester (CAPE-pNO2, a derivative of CAPE) on CMI and underlying mechanisms. SD rats were subjected to high-fat-cholesterol-diet (HFCD) and vitamin D3, and the H9c2 cells were treated with LPS to establish CMI model, followed by the respective treatment with saline, CAPE, or CAPE-pNO2. In vivo, CAPE-pNO2 could reduce serum lipid levels and improve impaired cardiac function and morphological changes. Data of related assays indicated that CAPE-pNO2 downregulated the expression of transforming growth factor-β1 (TGF-β1) and galectin-3 (Gal-3). Besides, CAPE-pNO2 decreased collagen deposition, the number of apoptotic cardiomyocytes, and some related downstream proteins of Gal-3 in the CMI rats. Interestingly, the effects of CAPE-pNO2 on TGF-β1, Gal-3, and other proteins expressed in the lung were consistent with that in the heart. In vitro, CAPE-pNO2 could attenuate the fibrosis, apoptosis, and inflammation by activating TGF-β1/Gal-3 pathway in LPS-induced H9c2 cell. However, CAPE-pNO2-mediated cardioprotection can be eliminated when treated with modified citrus pectin (MCP, an inhibitor of Gal-3). And in comparison, CAPE-pNO2 presented stronger effects than CAPE. This study indicates that CAPE-pNO2 may ameliorate CMI by suppressing fibrosis, inflammation, and apoptosis via the TGF-β1/Gal-3 pathway in vivo and in vitro.













Similar content being viewed by others
Data Availability
Data supporting the results of this research are available to the corresponding author on request.
Code Availability
Not applicable.
References
Orford, J.L., S. Kinlay, P. Ganz, and A.P. Selwyn. 2000. Treating ambulatory ischemia in coronary disease by manipulating the cell biology of atherosclerosis. Current Atherosclerosis Reports 2 (4): 321–326.
Chen, O.Y., Z.H. Ye, Z.Y. Cao, A. Manaenko, K. Ning, et al. 2016. Methane attenuates myocardial ischemia injury in rats through anti-oxidative, anti-apoptotic and anti-inflammatory actions. Free Radical Biology and Medicine 90: 1–11.
Palasubramaniam, J., X. Wang, and K. Peter. 2019. Myocardial Infarction-From Atherosclerosis to Thrombosis. Arteriosclerosis Thrombosis and Vascular Biology 39 (8): e176–e185.
Quinones, A., I. Lobach, G.A. Maduro, N.R. Smilowitz, and H.R. Reynolds. 2015. Diabetes and ischemic heart disease death in people age 25–54: A multiple-cause-of-death analysis based on over 400000 deaths from 1990 to 2008 in New York City. Clinical Cardiology 38 (2): 114–120.
Euler, G. 2015. Good and bad sides of TGF beta-signaling in myocardial infarction. Frontiers in Physiology 6: 66.
Zhang, X.G., Y. Wei, J. Jiang, L. Wang, H.Y. Liang, and C.B. Lei. 2020. Effect of TGF-β1 on myocardial cell apoptosis in rats with acute myocardial infarction via MAPK signaling pathway. European Review for Medical and Pharmacological Sciences 24 (3): 1350–1356.
Ge, Z.R., M.C. Xu, Y. Huang, C.J. Zhang, J. Lin, and C.W. Ruan. 2016. Cardioprotective effect of notoginsenoside R1 in a rabbit lung remote ischemic postconditioning model via activation of the TGF-beta 1/TAK1 signaling pathway. Experimental and Therapeutic Medicine 11 (6): 2341–2348.
Suchal, K., S. Malik, N. Gamad, R.K. Malhotra, S.N. Goyal, et al. 2016. Mangiferin protect myocardial insults through modulation of MAPK/TGF-beta pathways. European Journal of Pharmacology 776: 34–43.
Zhang, H., Y.C. Cui, K. Li, B.Q. Yang, X.P. Liu, et al. 2016. Glutamine protects cardiomyocytes from hypoxia/reoxygenation injury under high glucose conditions through inhibition of the transforming growth factor-beta1-Smad3 pathway. Archives of Biochemistry and Biophysics 596: 43–50.
Wang, L., and X.L. Guo. 2016. Molecular regulation of galectin-3 expression and therapeutic implication in cancer progression. Biomedicine & Pharmacotherapy 78: 165–171.
Carrasco-Sanchez, F.J., and M.I. Paez-Rubio. 2014. Review of the prognostic value of galectin-3 in heart failure focusing on clinical utility of repeated testing. Molecular Diagnosis & Therapy 18 (6): 599–604.
Blanda, V., U.M. Bracale, M.D. Di Taranto, and G. Fortunato. 2020. Galectin-3 in Cardiovascular Diseases. International Journal of Molecular Sciences 21 (23): 9232.
Li, M., Y. Yuan, K. Guo, Y. Lao, X. Huang, and L. Feng. 2020. Value of Galectin-3 in Acute Myocardial Infarction. American Journal of Cardiovascular Drugs 20 (4): 333–342.
Al-Salam, S., and S. Hashmi. 2018. Myocardial ischemia reperfusion injury: Apoptotic, inflammatory and oxidative stress role of galectin-3. Cellular Physiology and Biochemistry 50 (3): 1123–1139.
Zhang, M., K. Cheng, H. Chen, J. Tu, Y. Shen, et al. 2020. Galectin-3 knock down inhibits cardiac ischemia-reperfusion injury through interacting with bcl-2 and modulating cell apoptosis. Archives of Biochemistry and Biophysics 694: 108602.
Fulton, D.J.R., X. Li, Z. Bordan, Y. Wang, K. Mahboubi, et al. 2019. Galectin-3: A Harbinger of Reactive Oxygen Species, Fibrosis, and Inflammation in Pulmonary Arterial Hypertension. Antioxidants & Redox Signaling 31 (14): 1053–1069.
Agoston-Coldea, L., S. Lupu, D. Petrovai, T. Mocan, and E. Mousseaux. 2015. Correlations between echocardiographic parameters of right ventricular dysfunction and Galectin-3 in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Medical Ultrasonography 17 (4): 487–495.
Yoshimura, A., A. Gemma, Y. Hosoya, E. Komaki, Y. Hosomi, et al. 2003. Increased expression of the LGALS3 (Galectin-3) gene in human non-small-cell lung cancer. Genes Chromosomes & Cancer 37 (2): 159–164.
Lefkowitz, D.S., R. Sandhu, A. Kim, P. Wieczorek, A. Castellano, et al. 2018. The cardioprotective potential of caffeic acid phenethyl ester (CAPE) on H2O2-induced h9c2 cell damage compared to common anti-oxidants. Faseb Journal 32 (1): 841.5–841.5.
Castellano, A.J., T. Kuhn, S. Liu, K. Kucharski, J. Venditto, et al. 2016. The cardioprotective effects of caffeic acid phenethyl ester (CAPE) on myocardial ischemia/reperfusion (I/R) injury. Faseb Journal 30 (1): 1207.2–1207.2.
Du, Q., C.Z. Hao, J. Gou, X.L. Li, K.L. Zou, et al. 2016. Protective effects of p-nitro caffeic acid phenethyl ester on acute myocardial ischemia-reperfusion injury in rats. Experimental and Therapeutic Medicine 11 (4): 1433–1440.
Fan, L., Q.H. Xiao, L.W. Zhang, X.L. Wang, Q. Huang, et al. 2018. CAPE-pNO(2)attenuates diabetic cardiomyopathy through the NOX4/NF-kappaB pathway in STZ-induced diabetic mice. Biomedicine & Pharmacotherapy 108: 1640–1650.
Li, D.J., X.L. Wang, Q. Huang, S. Li, Y. Zhou, et al. 2018. Cardioprotection of CAPE-oNO(2) against myocardial ischemia/reperfusion induced ROS generation via regulating the SIRT1/eNOS/NF-kappa B pathway in vivo and in vitro. Redox Biology 15: 62–73.
Preidl, R.H.M., P. Moebius, M. Weber, K. Amann, F.W. Neukam, et al. 2015. Expression of transforming growth factor beta 1-related signaling proteins in irradiated vessels. Strahlentherapie und Onkologie 191 (6): 518–524.
Subramani, C., A. Rajakkannu, A. Rathinam, S. Gaidhani, and I. Raju. 2017. Anti-atherosclerotic activity of root bark of Premna integrifolia Linn in high fat diet induced atherosclerosis model rats. Journal of Pharmaceutical Analysis 7 (2): 123–128.
Zhang, H., H. Li, A. Ge, E. Guo, S. Liu, et al. 2018. Long non-coding RNA TUG1 inhibits apoptosis and inflammatory response in LPS-treated H9c2 cells by down-regulation of miR-29b. Biomedicine & Pharmacotherapy 101: 663–669.
Xu, J.J., C. Lin, T.T. Wang, P. Zhang, Z.J. Liu, et al. 2018. Ergosterol attenuates LPS-induced myocardial injury by modulating oxidative stress and apoptosis in rats. Cellular Physiology and Biochemistry 48 (2): 583–592.
Han, C.K., Y.C. Tien, D.J.Y. Hsieh, T.J. Ho, C.H. Lai, et al. 2017. Attenuation of the LPS-induced, ERK-mediated upregulation of fibrosis-related factors FGF-2, uPA, MMP-2, and MMP-9 by Carthamus tinctorius L in cardiomyoblasts. Environmental Toxicology 32 (3): 754–763.
Gou, J., X.F. Yao, H. Tang, K.L. Zou, Y.J. Liu, et al. 2016. Absorption properties and effects of caffeic acid phenethyl ester and its p-nitro-derivative on P-glycoprotein in Caco-2 cells and rats. Pharmaceutical Biology 54 (12): 296–2967.
Zhang, Z.H., D.D. Zhang, M.M. Dou, Z.B. Li, J. Zhang, et al. 2016. Dendrobium officinale Kimura et Migo attenuates diabetic cardiomyopathy through inhibiting oxidative stress, inflammation and fibrosis in streptozotocin-induced mice. Biomedicine & Pharmacotherapy 84: 1350–1358.
Severino, P., A. D’Amato, M. Pucci, F. Infusino, L.I. Birtolo, et al. 2020. Ischemic Heart Disease and Heart Failure: Role of Coronary Ion Channels. International Journal of Molecular Sciences 21 (9): 3167.
Rezende, P.C., F.F. Ribas, C.V. Serrano, and W. Hueb. 2019. Clinical significance of chronic myocardial ischemia in coronary artery disease patients. Journal of Thoracic Disease 11 (3): 1005–1015.
Neri, M., I. Riezzo, N. Pascale, C. Pomara, and E. Turillazzi. 2017. Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediators of Inflammation 7018393.
Caetano, J., and J.D. Alves. 2015. Heart rate and cardiovascular protection. European Journal of Internal Medicine 26 (4): 217–222.
Pascual Izco, M., R. Ramírez-Carracedo, I. Hernández Navarro, Á. Osorio Ruiz, B. Castejón Navarro, et al. 2020. Ivabradine in acute heart failure: Effects on heart rate and hemodynamic parameters in a randomized and controlled swine trial. Journal of Cardiology 27 (1): 62–71.
Sclarovsky, S. 2009. Upgrading the electrocardiogram in the 21st century. Journal of Electrocardiology 42 (1): 35–38.
Frampton, J., J.T. Devries, T.D. Welch, and B.J. Gersh. 2020. Modern management of ST-segment elevation myocardial infarction. Current Problems in Cardiology 45 (3): 100393.
Gramatikov, B., and V. Iyer. 2015. Intra-QRS spectral changes accompany ST segment changes during episodes of myocardial ischemia. Journal of Electrocardiology 48 (1): 115–122.
Liu, G.W., C. Ma, H.L. Yang, and P.Y. Zhang. 2017. Transforming growth factor beta and its role in heart disease. Experimental and Therapeutic Medicine 13 (5): 2123–2128.
Kang, Q., X.L. Li, M.Y. Yang, T. Fernando, and Z. Wan. 2018. Galectin-3 in patients with coronary heart disease and atrial fibrillation. Clinica Chimica Acta 478: 166–170.
Nguyen, M.N., Y. Su, D. Vizi, L. Fang, A.H. Ellims, W.B. Zhao, et al. 2018. Mechanisms responsible for increased circulating levels of galectin-3 in cardiomyopathy and heart failure. Scientific Reports 8: 523.
Wang, X.Y., Y.L. Wang, J.B. Zhang, X. Guan, M.G. Chen, Y.M. Li, et al. 2017. Galectin-3 contributes to vascular fibrosis in monocrotaline-induced pulmonary arterial hypertension rat model. Journal of Biochemical and Molecular Toxicology 31 (5): e21879.
Xiao, M., M. Zhang, M. Bie, X. Wang, J. Guo, et al. 2020. Galectin-3 induces atrial fibrosis by activating the TGF-β1/Smad pathway in patients with atrial fibrillation. Cardiology 145 (7): 446–455.
MacKinnon, A.C., M.A. Gibbons, S.L. Farnworth, H. Leffler, U.J. Nilsson, et al. 2012. Regulation of transforming growth factor-beta 1-driven lung fibrosis by galectin-3. American Journal of Respiratory and Critical Care Medicine 185 (5): 537–546.
Li, X., X. Tang, J.P. Lu, and S. Yuan. 2018. Therapeutic inhibition of galectin-3 improves cardiomyocyte apoptosis and survival during heart failure. Molecular Medicine Reports 17 (3): 4106–4112.
Kitazume-Taneike, R., M. Taneike, S. Omiya, T. Misaka, K. Nishida, et al. 2019. Ablation of Toll-like receptor 9 attenuates myocardial ischemia/reperfusion injury in mice. Biochemical and Biophysical Research Communications 515 (3): 442–447.
Xu, G.R., C. Zhang, H.X. Yang, J.H. Sun, Y. Zhang, et al. 2020. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-κB signaling pathway. Biomedicine & Pharmacotherapy 126: 110071.
Yamamoto, M., N. Kondo, M. Tashiro, K. Orihashi, K. Hanazaki, et al. 2018. Delayed production of reactive nitrogen species induces lung congestion after myocardial ischemia reperfusion. Journal of the American College of Surgeons 227 (4): 113.
Zhan, L.Y., Y. Zhang, W.T. Su, Q.X. Zhang, R. Chen, et al. 2018. The roles of autophagy in acute lung injury induced by myocardial ischemia reperfusion in diabetic rats. Journal of Diabetes Research 1–9.
Funding
This work was financially supported by the Fundamental research funds for the central university, China (SWU021003), Shanxi Zhaoyi Biological Co. Ltd., China (SWU2013130), and Skagen Animal Health Products (Shangqiu) Co., Ltd., China (2021073).
Author information
Authors and Affiliations
Contributions
Li Z, Zhang L, and Li B designed the project; Zhang L, Wan Q, and Han Y performed the experiments; Zhang L, Wan Q, Li Z, Li B, and Zhou Q wrote the manuscripts; Zhang L analyzed and interpreted data; and all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All the animal protocols were consistent with the National Institutes of Health (NIH) guidelines and approved by the Ethical Committee for Animals of Southwest University.
Consent to Participate
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
.
Rights and permissions
About this article
Cite this article
Wan, Q., Zhang, L., Zhou, Q. et al. Protection of CAPE-pNO2 Against Chronic Myocardial Ischemia by the TGF-Β1/Galectin-3 Pathway In Vivo and In Vitro. Inflammation 45, 1039–1058 (2022). https://doi.org/10.1007/s10753-021-01600-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01600-1