Abstract
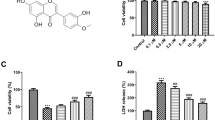
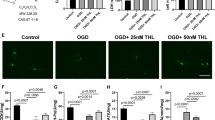
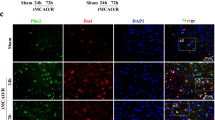
Baicalin has been reported to have ameliorative effects on nerve-induced hypoxic ischemia injury; however, its role in the NLRP3 inflammasome-dependent inflammatory response during cerebral ischemia-reperfusion remains unclear. To investigate the molecular mechanisms involved in baicalin alleviating cerebral ischemia-reperfusion injury, we investigated the AMPK signaling pathway which regulates NLRP3 inflammasome activity. SD rats were treated with baicalin at doses of 100 mg/kg and 200 mg/kg, respectively, after middle cerebral artery occlusion at 2 h and reperfusion for 24 h (MCAO/R). MCAO/R treatment significantly increased cerebral infarct volume, changed the ultrastructure of nerve cells, and activated the NLRP3 inflammasome, manifesting as significantly increased expression of NLRP3, ASC, cleaved caspase-1, IL-1β, and IL-18. Our results demonstrated that baicalin treatment effectively reversed these phenomena in a dose-dependent manner. Additionally, inhibition of NLRP3 expression was found to promote the neuroprotective effects of baicalin on cortical neurons. Furthermore, baicalin remarkably increased the expression of p-AMPK following oxygen glucose deprivation/reperfusion (OGD/R). The expression of the NLRP3 inflammasome was also increased when the AMPK pathway was blocked by compound C. Taken together, our findings reveal that baicalin reduces the activity of the NLRP3 inflammasome and consequently inhibits cerebral ischemia-reperfusion injury through activation of the AMPK signaling pathway.








Similar content being viewed by others
Abbreviations
- MCAO:
-
middle cerebral artery occlusion
- OGD:
-
oxygen and glucose deprivation
- LDH:
-
lactate dehydrogenase
- TEM:
-
transmission electron microscopy
- AMPK:
-
adenosine monophosphate–activated protein kinase
References
Gairolla, J., R. Kle, M. Modi, and D. Khurana. 2017. Leptin and adiponectin: pathophysiological role and possible therapeutic target of inflammation in ischemic stroke. Reviews in the Neurosciences 28: 295–306. https://doi.org/10.1515/revneuro-2016-0055.
Marta, D.B., M. Magdalena, G. Aleksandra, D.Z. Aneta, and S. Anna. 2017. The impact of ischemic cerebral stroke on the quality of life of patients based on clinical, social, and psychoemotional factors. Journal of Stroke and Cerebrovascular Diseases 26: 101–107. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.08.036.
Randolph, S.A. 2016. Ischemic Stroke. Workplace Health and Safety 64: 444. https://doi.org/10.1177/2165079916665400.
Sandercock, P.A.G., C. Counsell, M.C. Tseng, and E. Cecconi. 2014. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.
Zhang, Y.P., Y. Zhang, Z.B. Xiao, Y.B. Zhang, J. Zhang, Z.Q. Li, and Y.B. Zhu. 2018. CFTR prevents neuronal apoptosis following cerebral ischemia reperfusion via regulating mitochondrial oxidative stress. Journal of Molecular Medicine (Berlin, Germany) 96: 611–620. https://doi.org/10.1007/s00109-018-1649-2.
Röther, J. 2018. Neuroprotection does not work. Stroke 39: 523–524. https://doi.org/10.1161/STROKEAHA.107.494799.
Xie, F., H.B. Liu, and Y.H. Liu. 2020. Adult neurogenesis following ischemic stroke and implications for cell-based therapeutic approaches. World Neurosurgery 138: 474–480. https://doi.org/10.1016/j.wneu.2020.02.010.
Yang, J.L., Y.R. Yang, and S.D. Chen. 2019. The potential of drug repurposing combined with reperfusion therapy in cerebral ischemic stroke: a supplementary strategy to endovascular thrombectomy. Life Sciences 236: 116889. https://doi.org/10.1016/j.lfs.2019.116889.
Zgavc, T., A.G. Ceulemans, S. Sarre, Y. Michotte, and H.I. Said. 2011. Experimental and clinical use of therapeutic hypothermia for ischemic stroke: opportunities and limitations. Stroke Research and Treatment 689290: 1–9. https://doi.org/10.4061/2011/689290.
Nam, J.E., S.Y. Jo, C.W. Ahn, and Y.S. Kim. 2020. Baicalin attenuates fibrogenic process in human renal proximal tubular cells (HK−2) exposed to diabetic milieu. Life Sciences 254: 117742. https://doi.org/10.1016/j.lfs.2020.117742.
Jiang, M., Z.N. Li, and G.X. Zhu. 2020. Immunological regulatory effect of flavonoid baicalin on innate immune toll-like receptors. Pharmacological Research 158: 104890. https://doi.org/10.1016/j.phrs.2020.104890.
Ji, W.L., K. Liang, R. An, and X.H. Wang. 2019. Baicalin protects against ethanol-induced chronic gastritis in rats by inhibiting Akt/NF-κB pathway. Life Sciences 239: 117064. https://doi.org/10.1016/j.lfs.2019.117064.
Li, H., J. Hu, L. Ma, and Z. Yuan. 2010. Comprehensive study of baicalin down-regulating NOD2 receptor expression of neurons with oxygen-glucose deprivation in vitro and cerebral ischemia-reperfusion in vivo. European Journal of Pharmacology 15: 92–99. https://doi.org/10.1016/j.ejphar.2010.09.023.
Cao, Y., X. Mao, and C. Sun. 2011. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Research Bulletin 85 (6): 396–402. https://doi.org/10.1016/j.brainresbull.2011.05.002.
Zhu, H., Z. Wang, and Y. Xing. 2012. Baicalin reduces the permeability of the blood–brain barrier during hypoxia in vitro by increasing the expression of tight junction proteins in brain microvascular endothelial cells. Journal of Ethnopharmacology 141 (2): 714–720. https://doi.org/10.1016/j.jep.2011.08.063.
Woo, K.J., J.H. Lim, S. Suh, Y.K. Kwon, S.W. Shin, S.C. Kim, Y.H. Choi, J.W. Park, and T.K. Kwon. 2006. Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPbeta DNA-binding activity. Immunobiology 211: 359–368. https://doi.org/10.1016/j.imbio.2006.02.002.
Singh, D.P., and K. Chopra. 2014. Flavocoxid, dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase exhibits neuroprotection in a rat model of ischaemic stroke. Pharmacology, Biochemistry, and Behavior 120: 33–42. https://doi.org/10.1016/j.pbb.2014.02.006.
Lee, B., C. Lim, S. Lim, and S. Cho. 2019. Baicalin administered orally after ischemia/reperfusion alleviated brain injury in mice by inhibiting inflammation and edema. Natural Product Communications. https://doi.org/10.1177/1934578X19843032.
Cheng, F., Y. Lu, and X. Zhong. 2013. Baicalin’s therapeutic time window of neuroprotection during transient focal cerebral ischemia and its antioxidative effects in vitro and in vivo. Evidence-based Complementary and Alternative Medicine 120261: 1–11. https://doi.org/10.1155/2013/120261.
Cao, Y.G., X.Y. Mao, C.Y. Sun, P. Zheng, J.Q. Gao, X.R. Wang, D.Y. Min, H.L. Sun, and N. Xie. 2011. CaiJQ. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Research Bulletin 85 (6): 396–402. https://doi.org/10.1016/j.brainresbull.2011.05.002.
Li, S., X. Sun, and L. Xu. 2017. Baicalin attenuates In vivo and in vitro hyperglycemia-exacerbated ischemia/ reperfusion injury by regulating mitochondrial function in a manner dependent on AMPK. European Journal of Pharmacology 815: 118–126. https://doi.org/10.1016/j.ejphar.2017.07.041.
Tu, X.K., W.Z. Yang, and S.S. Shi. 2011. Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebralischemia. Inflammation 34 (5): 463–470. https://doi.org/10.1007/s10753-010-9254-8.
Zheng, W.X., F. Wang, X.L. Cao, H.Y. Pan, X.Y. Liu, X.M. Hu, and Y.Y. Sun. 2014. Baicalin protects PC-12 cells from oxidative stress induced by hydrogen peroxide via anti-apoptotic effects. Brain Injury 28: 227–234. https://doi.org/10.3109/02699052.2013.860469.
Zheng, W.X., X.L. Cao, F. Wang, J. Wang, T.Z. Ying, W. Xiao, Y. Zhang, H. Xing, W. Dong, S.Q. Xu, Z.L. Min, F.J. Wu, and X.M. Hu. 2015. Baicalin inhibiting cerebral ischemia/hypoxia-induced neuronal apoptosis via MRTF-A-mediated transactivity. European Journal of Pharmacology 767: 201–210. https://doi.org/10.1016/j.ejphar.2015.10.027.
Liu, J., T. Zhang, Y. Wang, et al. 2020. Baicalin ameliorates neuropathology in repeated cerebral ischemia-reperfusion injury model mice by remodeling the gut microbiota. Aging (Albany NY) 12 (4): 3791–3806. https://doi.org/10.18632/aging.102846.
Guo, H., J.B. Callaway, and J.P. Ting. 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature Medicine 21 (7): 677–687. https://doi.org/10.1038/nm.3893.
Walsh, J.G., D.A. Muruve, and C. Power. 2014. Inflammasomes in the CNS. Nature Reviews. Neuroscience 15 (2): 84–97. https://doi.org/10.1038/nrn3638.
Lakhan, S.E., A. Kirchgessner, and M. Hofer. 2009. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. Translational Medicine 7: 97–108. https://doi.org/10.1186/1479-5876-7-97.
Shabab, T., R. Khanabdali, S.Z. Moghadamtousi, H.A. Kadir, and G. Mohan. 2017. Neuroinflammation pathways: a general review. The International Journal of Neuroscience 127: 624–633. https://doi.org/10.1080/00207454.2016.1212854.
Yu, C., Q. He, J. Zheng, L.Y. Li, Y.H. Hou, and F.Z. Song. 2017. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. International Immunopharmacology 45: 74–78. https://doi.org/10.1016/j.intimp.2017.01.034.
Shah, M.A., D.J. Park, J.B. Kang, M.O. Kim, and P.O. Koh. 2019. Baicalin attenuates lipopolysaccharide-induced neuroinflammation in cerebral cortex of mice via inhibiting nuclear factor kappa B (NF-κB) activation. The Journal of Veterinary Medical Science 81: 1359–1367. https://doi.org/10.1292/jvms.19-0281.
Zhang, C.Y.Y., M.J. Zeng, L.P. Zhou, Y.Q. Li, F. Zhao, Z.Y. Shang, X.Y. Deng, Z.Q. Ma, Q. Fu, S.P. Ma, and R. Qu. 2018. Baicalin exerts neuroprotective effects via inhibiting activation of GSK3β/NF-κB/NLRP3 signal pathway in a rat model of depression. International Immunopharmacology 64: 175–182. https://doi.org/10.1016/j.intimp.2018.09.001.
Xue, X., X.J. Qu, Y. Yang, X.H. Sheng, F. Cheng, E.N. Jiang, J.H. Wang, W. Bu, and Z.P. Liu. 2010. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor κB p65 activation. Biochemical and Biophysical Research Communications 403 (3-4): 398–404. https://doi.org/10.1016/j.bbrc.2010.11.042.
Yang, Y.R., R.Y. Wang, and P.S. Wang. 2003. Early and late treadmill training after focal brain ischemia in rats. Neuroscience Letters 339: 91–94. https://doi.org/10.1016/s0304-3940(03)00010-7.
Schindelin, J., I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J.Y. Tinevez, D.J. White, V. Hartenstein, K. Eliceiri, P. Tomancak, and A. Cardona. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods 9: 676–682. https://doi.org/10.1038/nmeth.2019.
Gan, Y.M., D.L. Liu, C. Chen, W. Duan, Y.X. Yang, and J.R. Du. 2020. Phthalide derivative CD21 alleviates cerebral ischemia-induced neuroinflammation: involvement of microglial M2 polarization via AMPK activation. European Journal of Pharmacology 886: 5. https://doi.org/10.1016/j.ejphar.2020.173552.
Boese, A.C., J.P. Lee, and M.H. Hamblin. 2020. Neurovascular protection by peroxisome proliferator-activated receptor α in ischemic stroke. Experimental Neurology 331: 113323. https://doi.org/10.1016/j.expneurol.2020.113323.
Shen, Z., Y.R. Zheng, J.Y. Wu, Y. Chen, X.L. Wu, Y.T. Zhou, Y. Yuan, S.S. Lu, L. Jiang, Z.H. Qin, Z. Chen, W.W. Hu, and X.N. Zhang. 2017. PARK2-dependent mitophagy induced by acidic postconditioning protects against focal cerebral ischemia and extends the reperfusion window. Autophagy 13: 473–485. https://doi.org/10.1080/15548627.2016.1274596.
Sun, K., J.Y. Fan, and J.Y. Han. 2015. Ameliorating effects of traditional Chinese medicine preparation, Chinese Materia Medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharmaceutica Sinica B 5: 8–24. https://doi.org/10.1016/j.apsb.2014.11.002.
Yu, Z.H., M. Cai, X.T. Li, J.S. Zhang, T. Wu, F. Yang, W. Zhu, Y.J. Xiang, W. Zhang, J. Xiang, and D.F. Cai. 1685. Neuroprotective effects of tongxinluo on focal cerebral ischemia and reperfusion injury in rats associated with the activation of the MEK1/2/ERK1/2/p90RSK signaling pathway. Brain Research 2018: 9–18. https://doi.org/10.1016/j.brainres.2018.01.036.
Gong, L.L., Y.W. Tang, R. An, M.Y. Lin, L.J. Chen, and J. Du. 2017. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death & Disease 8: e3080. https://doi.org/10.1038/cddis.2017.465.
Zhou, L.Y., Y. Wang, K. Wang, J. Wang, A.J. Ma, and X.D. Pan. 2019. Potential therapeutic drugs for ischemic stroke based on bioinformatics analysis. The International Journal of Neuroscience 129: 1098–1102. https://doi.org/10.1080/00207454.2019.1634072.
Williams, E.I., R.D. Betterton, T.P. Davis, and P.T. Ronaldson. 2020. Transporter-mediated delivery of small molecule drugs to the brain: a critical mechanism that can advance therapeutic development for ischemic stroke. Pharmaceutics 12: 154. https://doi.org/10.3390/pharmaceutics12020154.
Powers, W.J., A.A. Rabinstein, T. Ackerson, O.M. Adeoye, N.C. Bambakidis, K. Becker, J. Biller, M. Brown, B.M. Demaerschalk, B. Hoh, E.C. Jauch, C.S. Kidwell, T.M. Leslie-Mazwi, B. Ovbiagele, P.A. Scott, K.N. Sheth, A.M. Southerland, D.V. Summers, D.L. Tirschwell, and on behalf of the American Heart Association Stroke Council. 2019. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 50 (12): e344–e418. https://doi.org/10.1161/STR.0000000000000211.
Powers, W.J., A.A. Rabinstein, T. Ackerson, O.M. Adeoye, N.C. Bambakidis, K. Becker, J. Biller, M. Brown, B.M. Demaerschalk, B. Hoh, E.C. Jauch, C.S. Kidwell, T.M. Leslie-Mazwi, B. Ovbiagele, P.A. Scott, K.N. Sheth, A.M. Southerland, D.V. Summers, D.L. Tirschwell, and American Heart Association Stroke Council. 2018. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49 (3): e46–e110. https://doi.org/10.1161/STR.0000000000000158.
Zhang, A.P., Y.Y. Zhang, A.F. Liu, K. Wang, C. Li, Y.E. Liu, Y.Q. Zhang, J. Zhou, J. Lv, and W.J. Jiang. 2020. Molecular mechanism of long-term neuroprotective effects of gradual flow restoration on cerebral ischemia reperfusion injury in MCAO rats. Journal of Stroke and Cerebrovascular Diseases 29: 105041. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105041.
Wu, B., M. Liu, H. Liu, W. Li, S. Tan, S. Zhang, and Y. Fang. 2007. Meta-analysis of traditional Chinese patent medicine for ischemic stroke. Stroke 38 (6): 1973–1979. https://doi.org/10.1161/STROKEAHA.106.473165.
Zhao, H., C.D. Li, L.N. Li, J.Y. Liu, Y.H. Gao, H. Mu, D.H. Chen, A.P. Lu, Y.Y. Ren, and Z.H. Li. 2020. Baicalin alleviates bleomycin-induced pulmonary fibrosis and fibroblast proliferation in rats via the PI3K/AKT signaling pathway. Molecular Medicine Reports 21: 2321–2334. https://doi.org/10.3892/mmr.2020.11046.
Miao, Y., M. Ishfaq, Y. Liu, Z. Wu, J. Wang, R. Li, F. Qian, L. Ding, and J. Lia. 2020. Baicalin attenuates endometritis in a rabbit model induced by infection with Escherichia coli and Staphylococcus aureus via NF-κB and JNK signaling pathways. Domestic Animal Endocrinology 106508: 106508. https://doi.org/10.1016/j.domaniend.2020.106508.
Fu, S.L., J. Liu, J.F. Xu, S.L. Zuo, Y.F. Zhang, L. Guo, Y.S. Qiu, C. Ye, Y. Liu, Z.Y. Wu, Y.Q. Hou, and C.A.A. Hu. 2020. The effect of baicalin on microRNA expression profiles in porcine aortic vascular endothelial cells infected by Haemophilus Parasuis. Molecular and Cellular Biochemistry 472 (1-2): 45–56. https://doi.org/10.1007/s11010-020-03782-y.
Liang, W., X.B. Huang, and W.Q. Chen. 2017. The effects of baicalin and baicalein on cerebral ischemia: a review. Aging and Disease 8: 850–867. https://doi.org/10.14336/AD.2017.0829.
Song, X.R., Z.X. Gong, K.L. Liu, J.P. Kou, B.L. Liu, and K. Liu. 2020. Baicalin combats glutamate excitotoxicity via protecting glutamine synthetase from ROS-induced 20S proteasomal degradation. Redox Biology 34: 101559. https://doi.org/10.1016/j.redox.2020.101559.
Sowndhararajan, K., P. Deepa, M. Kim, S.J. Park, and S. Kim. 2018. Neuroprotective and cognitive enhancement potentials of baicalin: a review. Brain Sciences 8: 104. https://doi.org/10.3390/brainsci8060104.
Li, N., L. Feng, Y. Tan, et al. 2018. Preparation, characterization, pharmacokinetics and biodistribution of baicalin-loaded liposome on cerebral ischemia-reperfusion after i.v. administration in rats, Molecules. 23 (7). https://doi.org/10.3390/molecules23071747.
Wei, Y.M., J.M. Guo, X.L. Zheng, et al. 2014. Preparation, pharmacokinetics and biodistribution of baicalin-loaded liposomes. International Journal of Nanomedicine 9: 3623–3630. https://doi.org/10.2147/IJN.S66312.
Bennion, D.M., U.M. Steckelings, and C. Sumners. 2018. Neuroprotection via AT2 receptor agonists in ischemic stroke. Clinical Science (London, England) 132: 1055–1067. https://doi.org/10.1042/CS20171549.
Heijden, T., E. Kritikou, W. Venema, J. Duijn, P.J. Santbrink, B. Slütter, A.C. Foks, I. Bot, and J. Kuiper. 2017. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arteriosclerosis, Thrombosis, and Vascular Biology 37: 1457–1461. https://doi.org/10.1161/ATVBAHA.117.309575.
Qiu, Z., S.Q. Lei, B. Zhao, Y. Wu, W.T. Su, M. Liu, Q.T. Meng, B. Zhou, Y. Leng, and Z.Y. Xia. 2017. NLRP3 Inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxidative Medicine and Cellular Longevity 9743280: 1–17. https://doi.org/10.1155/2017/9743280.
An, P.P., J. Xie, S. Qiu, Y.J. Liu, J.N. Wang, X.H. Xiu, L. Li, and M. Tang. 2019. Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sciences 232: 116599. https://doi.org/10.1016/j.lfs.2019.116599.
Elliott, E.I., and F.S. Sutterwala. 2015. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunological Reviews 265: 35–52. https://doi.org/10.1111/imr.12286.
Jo, E.K., J.K. Kim, D.M. Shin, and C. Sasakawa. 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cellular & Molecular Immunology 13: 148–159. https://doi.org/10.1038/cmi.2015.95.
Chen, G.H., X.L. Li, Y.Q. Deng, F.M. Zhou, W.Q. Zou, W.X. Jiang, S.Q. Shangguan, and Z.N. Lu. 2019. The molecular mechanism of EPO regulates the angiogenesis after cerebral ischemia through AMPK-KLF2 signaling pathway. Critical Reviews in Eukaryotic Gene Expression 29: 105–112. https://doi.org/10.1615/CritRevEukaryotGeneExpr.2019029018.
Narayana, P.R., G.F.G. Aguilar, E.R. Mónica, and A. Penélope. 2018. Current evidence for AMPK activation involvement on resveratrol-induced neuroprotection in cerebral ischemia. Nutritional Neuroscience 21: 229–247. https://doi.org/10.1080/1028415X.2017.1284361.
Cordero, M.D., M.R. Williams, and B. Ryffel. 2018. AMP-activated protein kinase regulation of the NLRP3 inflammasome during aging. Trends in Endocrinology and Metabolism 29 (1): 8–17. https://doi.org/10.1016/j.tem.2017.10.009.
Funding
The study was supported by the National Natural Science Foundation of China (81801307) and Wuhan Municipal Health Commission 2018 Medical Scientific Research Project (WX18C28).
Author information
Authors and Affiliations
Contributions
The study was designed and the data were audited by Wen-Xia Zheng and Fang-Jian Wu; the cell experiment was operated by Qian-Rui Zhang and Sheng Zhao; the animal experiment was operated by Wen-Qi He and Jin-Xin Jia; the manuscript was prepared and the data were studied by Xiao-Lu Cao. The final manuscript was read and approved by all authors.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All Sprague-Dawley rats used in this study were housed in a specific pathogen-free facility, received humanistic care, and were used according to the animal care regulations of Wuhan University of Science and Technology.
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Highlight
1. Baicalin attenuates injury induced by cerebral ischemia-reperfusion through inhibits NLRP3 inflammasome activity.
2. NLRP3 is a key factor in the neuroprotective effect of baicalin during cerebral ischemia-reperfusion.
3. Baicalin regulates NLRP3 inflammasome activity via the AMPK signaling pathway.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, WX., He, WQ., Zhang, QR. et al. Baicalin Inhibits NLRP3 Inflammasome Activity Via the AMPK Signaling Pathway to Alleviate Cerebral Ischemia-Reperfusion Injury. Inflammation 44, 2091–2105 (2021). https://doi.org/10.1007/s10753-021-01486-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01486-z