Abstract
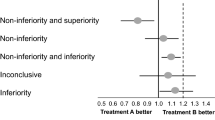
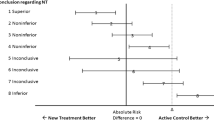
Non-inferiority trials are used to test if a novel intervention is not worse than a standard treatment by more than a prespecified amount, the non-inferiority margin (ΔNI). The ΔNI indicates the amount of efficacy loss in the primary outcome that is acceptable in exchange for non-efficacy benefits in other outcomes. However, non-inferiority designs are sometimes used when non-efficacy benefits are absent. Without non-efficacy benefits, loss in efficacy cannot be easily justified. Further, non-efficacy benefits are scarcely defined or considered by trialists when determining the magnitude of and providing justification for the non-inferiority margin. This is problematic as the importance of a treatment’s non-efficacy benefits are critical to understanding the results of a non-inferiority study. Here we propose the routine reporting in non-inferiority trial protocols and publications of non-efficacy benefits of the novel intervention along with the reporting of non-inferiority margins and their justification. The justification should include the specific trade-off between the accepted loss in efficacy (ΔNI) and the non-efficacy benefits of the novel treatment and should describe whether patients and other relevant stakeholders were involved in the definition of the ΔNI.

Similar content being viewed by others
Change history
28 November 2021
In the title of the article the word “on-inferiority” has been changed to "non inferiority"
References
Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues. Ann Intern Med. 2000;133(6):455–63.
Kaul S, Diamond GA. Good enough: a primer on the analysis and interpretation of noninferiority trials. Ann Intern Med. 2006;145(1):62–9.
Mauri L, D’Agostino RB Sr. Challenges in the design and interpretation of noninferiority trials. N Engl J Med. 2017;377(14):1357–67.
Acuna SA, Dossa F, Baxter NN. Frequency of misinterpretation of inconclusive noninferiority trials: the case of the laparoscopic vs open resection for rectal cancer trials. JAMA Surg. 2018;154:90.
Acuna SA, Chesney TR, Baxter NN. Incorporating patient preferences in noninferiority trials. JAMA. 2019;322(4):305–6.
Yu J, Huang C, Sun Y, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 2019;321(20):1983–92.
Alexander E, Goldberg L, Das AF, et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: the LEAP 2 randomized clinical trial. JAMA. 2019;322(17):1661–71.
Rehal S, Morris TP, Fielding K, Carpenter JR, Phillips pp. . Non-inferiority trials: are they inferior? A systematic review of reporting in major medical journals. BMJ open. 2016;6(10):e012594.
Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. CONSORT Group ft. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. Jama. 2012;308(24):2594–604.
Gopal AD, Desai NR, Tse T, Ross JS. Reporting of noninferiority trials in ClinicalTrials gov. and corresponding publications. Jama. 2015;313(11):1163–5.
Le Henanff A, Giraudeau B, Baron G, Ravaud P. Quality of reporting of noninferiority and equivalence randomized trials. JAMA. 2006;295(10):1147–51.
Hofheinz R-D, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–88.
Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. The Lancet. 2018;391(10139):2525–36.
Abt D, Hechelhammer L, Müllhaupt G, et al. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ. 2018;361.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Acuna, S.A., Dossa, F. & Chesney, T.R. Improving the reporting of non-inferiority trials by incorporating non-efficacy benefits: not all non-inferiority trials are created equal. Eur J Epidemiol 36, 1097–1101 (2021). https://doi.org/10.1007/s10654-021-00791-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-021-00791-z