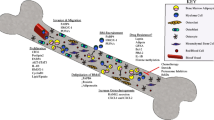
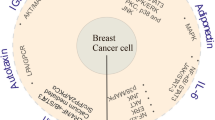
Abstract
Bone marrow adipose tissues (BMATs) and their main cellular component, bone marrow adipocytes (BMAds), are found within the bone marrow (BM), which is a niche for the development of hematological malignancies as well as bone metastasis from solid tumors such as breast and prostate cancers. In humans, BMAds are present within the hematopoietic or “red” BMAT and in the “yellow” BMAT where they are more densely packed. BMAds are emerging as new actors in tumor progression; however, there are many outstanding questions regarding their precise role. In this review, we summarized our current knowledge regarding the development, distribution, and regulation by external stimuli of the BMATs in mice and humans and addressed how obesity could affect these traits. We then discussed the specific metabolic phenotype of BMAds that appear to be different from “classical” white adipocytes, since they are devoid of lipolytic function. According to this characterization, we presented how tumor cells affect the in vitro and in vivo phenotype of BMAds and the signals emanating from BMAds that are susceptible to modulate tumor behavior with a specific emphasis on their metabolic crosstalk with cancer cells. Finally, we discussed how obesity could affect this crosstalk. Deciphering the role of BMAds in tumor progression would certainly lead to the identification of new targets in oncology in the near future.



Similar content being viewed by others
References
Zwick, R. K., Guerrero-Juarez, C. F., Horsley, V., & Plikus, M. V. (2018). Anatomical, physiological, and functional diversity of adipose tissue. Cell Metabolism, 27(1), 68–83. https://doi.org/10.1016/j.cmet.2017.12.002
Duong, M. N., Geneste, A., Fallone, F., Li, X., Dumontet, C., & Muller, C. (2017). The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget, 8(34), 57622–57641. https://doi.org/10.18632/oncotarget.18038
Lengyel, E., Makowski, L., DiGiovanni, J., & Kolonin, M. G. (2018). Cancer as a matter of fat: The crosstalk between adipose tissue and tumors. Trends in Cancer, 4(5), 374–384. https://doi.org/10.1016/j.trecan.2018.03.004
Attané, C., & Muller, C. (2020). Drilling for oil: Tumor-surrounding adipocytes fueling canceR. Trends in Cancer, 6(7), 593–604. https://doi.org/10.1016/j.trecan.2020.03.001
Morigny, P., Boucher, J., Arner, P., & Langin, D. (2021). Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nature Reviews. Endocrinology, 17(5), 276–295. https://doi.org/10.1038/s41574-021-00471-8
Ouchi, N., Parker, J. L., Lugus, J. J., & Walsh, K. (2011). Adipokines in inflammation and metabolic disease. Nature Reviews. Immunology, 11(2), 85–97. https://doi.org/10.1038/nri2921
Fasshauer, M., & Blüher, M. (2015). Adipokines in health and disease. Trends in Pharmacological Sciences, 36(7), 461–470. https://doi.org/10.1016/j.tips.2015.04.014
Calle, E. E., Rodriguez, C., Walker-Thurmond, K., & Thun, M. J. (2003). Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England Journal of Medicine, 348(17), 1625–1638. https://doi.org/10.1056/NEJMoa021423
Renehan, A. G., Zwahlen, M., & Egger, M. (2015). Adiposity and cancer risk: New mechanistic insights from epidemiology. Nature Reviews. Cancer, 15(8), 484–498. https://doi.org/10.1038/nrc3967
Kahn, C. R., Wang, G., & Lee, K. Y. (2019). Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. The Journal of Clinical Investigation, 129(10), 3990–4000. https://doi.org/10.1172/JCI129187
Nieman, K. M., Kenny, H. A., Penicka, C. V., Ladanyi, A., Buell-Gutbrod, R., Zillhardt, M. R., … & Lengyel, E. (2011). Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine, 17(11), 1498–1503. https://doi.org/10.1038/nm.2492
Nieman, K. M., Romero, I. L., Van Houten, B., & Lengyel, E. (2013). Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica Et Biophysica Acta, 1831(10), 1533–1541. https://doi.org/10.1016/j.bbalip.2013.02.010
Ye, H., Adane, B., Khan, N., Sullivan, T., Minhajuddin, M., Gasparetto, M., … & Jordan, C. T. (2016). Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell, 19(1), 23–37. https://doi.org/10.1016/j.stem.2016.06.001
Macedo, F., Ladeira, K., Pinho, F., Saraiva, N., Bonito, N., Pinto, L., & Goncalves, F. (2017). Bone metastases: An overview. Oncology Reviews, 11(1), 321. https://doi.org/10.4081/oncol.2017.321
Scheller, E. L., Cawthorn, W. P., Burr, A. A., Horowitz, M. C., & MacDougald, O. A. (2016). Marrow adipose tissue: Trimming the fat. Trends in endocrinology and metabolism: TEM, 27(6), 392–403. https://doi.org/10.1016/j.tem.2016.03.016
Cawthorn, W. P., & Scheller, E. L. (2017). Editorial: Bone marrow adipose tissue: Formation, Function, and impact on health and disease. Frontiers in Endocrinology, 8, 112. https://doi.org/10.3389/fendo.2017.00112
Li, Z., Hardij, J., Bagchi, D. P., Scheller, E. L., & MacDougald, O. A. (2018). Development, regulation, metabolism and function of bone marrow adipose tissues. Bone, 110, 134–140. https://doi.org/10.1016/j.bone.2018.01.008
Neumann E. (1882). Das Gesetz der Verbreitung des Gelben und rotten Knochenmaarkes. Zentralbl Med Wissensch., pp. 321–323.
Tavassoli, M. (1976). Marrow adipose cells. Histochemical identification of labile and stable components. Archives of Pathology & Laboratory Medicine, 100(1), 16–18.
Scheller, E. L., Doucette, C. R., Learman, B. S., Cawthorn, W. P., Khandaker, S., Schell, B., … & MacDougald, O. A. (2015). Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nature Communications, 6(1), 7808. https://doi.org/10.1038/ncomms8808
Styner, M., Pagnotti, G. M., McGrath, C., Wu, X., Sen, B., Uzer, G., … & Rubin, J. (2017). Exercise decreases marrow adipose tissue through ß-oxidation in obese running mice: Exercise decreases mat in obese mice. Journal of Bone and Mineral Research, 32(8), 1692–1702. https://doi.org/10.1002/jbmr.3159
Scheller, E. L., Khandaker, S., Learman, B. S., Cawthorn, W. P., Anderson, L. M., Pham, H. A., … & MacDougald, O. A. (2019). Bone marrow adipocytes resist lipolysis and remodeling in response to β-adrenergic stimulation. Bone, 118, 32–41. https://doi.org/10.1016/j.bone.2018.01.016
Robles, H., Park, S., Joens, M. S., Fitzpatrick, J. A. J., Craft, C. S., & Scheller, E. L. (2019). Characterization of the bone marrow adipocyte niche with three-dimensional electron microscopy. Bone, 118, 89–98. https://doi.org/10.1016/j.bone.2018.01.020
Tratwal, J., Labella, R., Bravenboer, N., Kerckhofs, G., Douni, E., Scheller, E. L., … Naveiras, O. (2020). Reporting guidelines, review of methodological standards, and challenges toward harmonization in bone marrow adiposity research. Report of the Methodologies Working Group of the International Bone Marrow Adiposity Society. Frontiers in Endocrinology, 11, 65. https://doi.org/10.3389/fendo.2020.00065
Cawthorn, W. P., Scheller, E. L., Learman, B. S., Parlee, S. D., Simon, B. R., Mori, H., … & MacDougald, O. A. (2014). Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metabolism, 20(2), 368–375. https://doi.org/10.1016/j.cmet.2014.06.003
Justesen, J., Stenderup, K., Ebbesen, E. N., Mosekilde, L., Steiniche, T., & Kassem, M. (2001). Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology, 2(3), 165–171. https://doi.org/10.1023/a:1011513223894
Kricun, M. E. (1985). Red-yellow marrow conversion: Its effect on the location of some solitary bone lesions. Skeletal Radiology, 14(1), 10–19. https://doi.org/10.1007/BF00361188
Blebea, J. S., Houseni, M., Torigian, D. A., Fan, C., Mavi, A., Zhuge, Y., … & Alavi, A. (2007). Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Seminars in Nuclear Medicine, 37(3), 185–194. https://doi.org/10.1053/j.semnuclmed.2007.01.002
Kugel, H., Jung, C., Schulte, O., & Heindel, W. (2001). Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. Journal of magnetic resonance imaging: JMRI, 13(2), 263–268. https://doi.org/10.1002/1522-2586(200102)13:2%3c263::aid-jmri1038%3e3.0.co;2-m
Pansini, V., Monnet, A., Salleron, J., Hardouin, P., Cortet, B., & Cotten, A. (2014). 3 Tesla (1) H MR spectroscopy of hip bone marrow in a healthy population, assessment of normal fat content values and influence of age and sex. Journal of magnetic resonance imaging: JMRI, 39(2), 369–376. https://doi.org/10.1002/jmri.24176
Griffith, J. F., Yeung, D. K. W., Ma, H. T., Leung, J. C. S., Kwok, T. C. Y., & Leung, P. C. (2012). Bone marrow fat content in the elderly: A reversal of sex difference seen in younger subjects. Journal of magnetic resonance imaging: JMRI, 36(1), 225–230. https://doi.org/10.1002/jmri.23619
Suchacki, K. J., Tavares, A. A. S., Mattiucci, D., Scheller, E. L., Papanastasiou, G., Gray, C., … & Cawthorn, W. P. (2020). Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nature Communications, 11(1), 3097. https://doi.org/10.1038/s41467-020-16878-2
Lucas, S., Tencerova, M., von der Weid, B., Andersen, T. L., Attané, C., Behler-Janbeck, F., … & van der Eerden, B. C. J. (2021). Guidelines for biobanking of bone marrow adipose tissue and related cell types: Report of the Biobanking Working Group of the International Bone Marrow Adiposity Society. Frontiers in Endocrinology, 12, 744527. https://doi.org/10.3389/fendo.2021.744527
Attané, C., Estève, D., Moutahir, M., Reina, N., & Muller, C. (2021). A protocol for human bone marrow adipocyte isolation and purification. STAR protocols, 2(3), 100629. https://doi.org/10.1016/j.xpro.2021.100629
Attané, C., Estève, D., Chaoui, K., Iacovoni, J. S., Corre, J., Moutahir, M., … & Muller, C. (2020). Human bone marrow is comprised of adipocytes with specific lipid metabolism. Cell Reports, 30(4), 949-958.e6. https://doi.org/10.1016/j.celrep.2019.12.089
Zechner, R. (2015). FAT FLUX: Enzymes, regulators, and pathophysiology of intracellular lipolysis. EMBO molecular medicine, 7(4), 359–362. https://doi.org/10.15252/emmm.201404846
Devlin, M. J., Cloutier, A. M., Thomas, N. A., Panus, D. A., Lotinun, S., Pinz, I., … & Bouxsein, M. L. (2010). Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 25(9), 2078–2088. https://doi.org/10.1002/jbmr.82
Bathija, A., Davis, S., & Trubowitz, S. (1979). Bone marrow adipose tissue: Response to acute starvation. American Journal of Hematology, 6(3), 191–198. https://doi.org/10.1002/ajh.2830060303
Tavassoli, M. (1974). Differential response of bone marrow and extramedullary adipose cells to starvation. Experientia, 30(4), 424–425. https://doi.org/10.1007/BF01921701
Abella, E., Feliu, E., Granada, I., Millá, F., Oriol, A., Ribera, J. M., … & Rozman, C. (2002). Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. American Journal of Clinical Pathology, 118(4), 582–588. https://doi.org/10.1309/2Y7X-YDXK-006B-XLT2
Bredella, M. A., Torriani, M., Ghomi, R. H., Thomas, B. J., Brick, D. J., Gerweck, A. V., … & Miller, K. K. (2011). Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity, 19(1), 49–53. https://doi.org/10.1038/oby.2010.106
Cawthorn, W. P., Scheller, E. L., Parlee, S. D., Pham, H. A., Learman, B. S., Redshaw, C. M. H., … & MacDougald, O. A. (2016). Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology, 157(2), 508–521. https://doi.org/10.1210/en.2015-1477
Ghali, O., Al Rassy, N., Hardouin, P., & Chauveau, C. (2016). Increased bone marrow adiposity in a context of energy deficit: The tip of the iceberg? Frontiers in Endocrinology, 7, 125. https://doi.org/10.3389/fendo.2016.00125
Bartelt, A., Koehne, T., Tödter, K., Reimer, R., Müller, B., Behler-Janbeck, F., … & Niemeier, A. (2017). Quantification of bone fatty acid metabolism and its regulation by adipocyte lipoprotein lipase. International Journal of Molecular Sciences, 18(6), E1264. https://doi.org/10.3390/ijms18061264
Ojala, R., Motiani, K. K., Ivaska, K. K., Arponen, M., Eskelinen, J.-J., Virtanen, K. A., & Hannukainen, J. C. (2020). Bone marrow metabolism is impaired in insulin resistance and improves after exercise training. The Journal of Clinical Endocrinology and Metabolism, 105(12), dgaa516. https://doi.org/10.1210/clinem/dgaa516
Kozubík, A., Sedláková, A., Pospísil, M., & Petrásek, R. (1988). In vivo studies of the relationship between the activation of lipid metabolism, postirradiation bone marrow cell proliferation and radioresistance of mice. General Physiology and Biophysics, 7(3), 293–302.
Pham, T. T., Ivaska, K. K., Hannukainen, J. C., Virtanen, K. A., Lidell, M. E., Enerbäck, S., … Kiviranta, R. (2020). Human bone marrow adipose tissue is a metabolically active and insulin-sensitive distinct fat depot. The Journal of Clinical Endocrinology and Metabolism, 105(7), dgaa216. https://doi.org/10.1210/clinem/dgaa216
Zechner, R., Madeo, F., & Kratky, D. (2017). Cytosolic lipolysis and lipophagy: Two sides of the same coin. Nature Reviews. Molecular Cell Biology, 18(11), 671–684. https://doi.org/10.1038/nrm.2017.76
Sulston, R. J., & Cawthorn, W. P. (2016). Bone marrow adipose tissue as an endocrine organ: Close to the bone? Hormone Molecular Biology and Clinical Investigation, 28(1), 21–38. https://doi.org/10.1515/hmbci-2016-0012
Scheller, E. L., Burr, A. A., MacDougald, O. A., & Cawthorn, W. P. (2016). Inside out: Bone marrow adipose tissue as a source of circulating adiponectin. Adipocyte, 5(3), 251–269. https://doi.org/10.1080/21623945.2016.1149269
Uchihashi, K., Aoki, S., Shigematsu, M., Kamochi, N., Sonoda, E., Soejima, H., … & Toda, S. (2010). Organotypic culture of human bone marrow adipose tissue. Pathology International, 60(4), 259–267. https://doi.org/10.1111/j.1440-1827.2010.02511.x
Miggitsch, C., Meryk, A., Naismith, E., Pangrazzi, L., Ejaz, A., Jenewein, B., … & Grubeck-Loebenstein, B. (2019). Human bone marrow adipocytes display distinct immune regulatory properties. eBioMedicine, 46, 387–398. https://doi.org/10.1016/j.ebiom.2019.07.023
Liu, L.-F., Shen, W.-J., Ueno, M., Patel, S., & Kraemer, F. B. (2011). Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics, 12, 212. https://doi.org/10.1186/1471-2164-12-212
Mattiucci, D., Maurizi, G., Izzi, V., Cenci, L., Ciarlantini, M., Mancini, S., … & Poloni, A. (2018). Bone marrow adipocytes support hematopoietic stem cell survival. Journal of Cellular Physiology, 233(2), 1500–1511. https://doi.org/10.1002/jcp.26037
Ghaben, A. L., & Scherer, P. E. (2019). Adipogenesis and metabolic health. Nature Reviews. Molecular Cell Biology, 20(4), 242–258. https://doi.org/10.1038/s41580-018-0093-z
Lange, M., Angelidou, G., Ni, Z., Criscuolo, A., Schiller, J., Blüher, M., & Fedorova, M. (2021). AdipoAtlas: A reference lipidome for human white adipose tissue. Cell Reports. Medicine, 2(10), 100407. https://doi.org/10.1016/j.xcrm.2021.100407
Yew Tan, C., Virtue, S., Murfitt, S., Roberts, L. D., Robert, L. D., Phua, Y. H., … & Vidal-Puig, A. (2015). Adipose tissue fatty acid chain length and mono-unsaturation increases with obesity and insulin resistance. Scientific Reports, 5, 18366. https://doi.org/10.1038/srep18366
Laurencikiene, J., Skurk, T., Kulyté, A., Hedén, P., Aström, G., Sjölin, E., … & Arner, P. (2011). Regulation of lipolysis in small and large fat cells of the same subject. The Journal of Clinical Endocrinology and Metabolism, 96(12), E2045-2049. https://doi.org/10.1210/jc.2011-1702
Skurk, T., Alberti-Huber, C., Herder, C., & Hauner, H. (2007). Relationship between adipocyte size and adipokine expression and secretion. The Journal of Clinical Endocrinology and Metabolism, 92(3), 1023–1033. https://doi.org/10.1210/jc.2006-1055
Reilly, S. M., & Saltiel, A. R. (2017). Adapting to obesity with adipose tissue inflammation. Nature Reviews. Endocrinology, 13(11), 633–643. https://doi.org/10.1038/nrendo.2017.90
Liu, L.-F., Shen, W.-J., Ueno, M., Patel, S., Azhar, S., & Kraemer, F. B. (2013). Age-related modulation of the effects of obesity on gene expression profiles of mouse bone marrow and epididymal adipocytes. PLoS ONE, 8(8), e72367. https://doi.org/10.1371/journal.pone.0072367
Doucette, C. R., Horowitz, M. C., Berry, R., MacDougald, O. A., Anunciado-Koza, R., Koza, R. A., & Rosen, C. J. (2015). A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. Journal of Cellular Physiology, 230(9), 2032–2037. https://doi.org/10.1002/jcp.24954
Lecka-Czernik, B., Stechschulte, L. A., Czernik, P. J., & Dowling, A. R. (2015). High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Molecular and Cellular Endocrinology, 410, 35–41. https://doi.org/10.1016/j.mce.2015.01.001
Tencerova, M., Figeac, F., Ditzel, N., Taipaleenmäki, H., Nielsen, T. K., & Kassem, M. (2018). High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 33(6), 1154–1165. https://doi.org/10.1002/jbmr.3408
Bredella, M. A., Gill, C. M., Gerweck, A. V., Landa, M. G., Kumar, V., Daley, S. M., … & Miller, K. K. (2013). Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology, 269(2), 534–541. https://doi.org/10.1148/radiol.13130375
Yu, E. W., Greenblatt, L., Eajazi, A., Torriani, M., & Bredella, M. A. (2017). Marrow adipose tissue composition in adults with morbid obesity. Bone, 97, 38–42. https://doi.org/10.1016/j.bone.2016.12.018
Fazeli, P. K., Bredella, M. A., Pachon-Peña, G., Zhao, W., Zhang, X., Faje, A. T., … & Klibanski, A. (2021). The dynamics of human bone marrow adipose tissue in response to feeding and fasting. JCI Insight, 6(12), e138636. https://doi.org/10.1172/jci.insight.138636
Bredella, M. A., Buckless, C., Fazeli, P. K., Rosen, C. J., Torriani, M., Klibanski, A., & Miller, K. K. (2021). Bone marrow adipose tissue composition following high-caloric feeding and fasting. Bone, 152, 116093. https://doi.org/10.1016/j.bone.2021.116093
Singhal, V., Torre Flores, L. P., Stanford, F. C., Toth, A. T., Carmine, B., Misra, M., & Bredella, M. A. (2018). Differential associations between appendicular and axial marrow adipose tissue with bone microarchitecture in adolescents and young adults with obesity. Bone, 116, 203–206. https://doi.org/10.1016/j.bone.2018.08.009
Singhal, V., Bose, A., Liang, Y., Srivastava, G., Goode, S., Stanford, F. C., … & Bredella, M. A. (2019). Marrow adipose tissue in adolescent girls with obesity. Bone, 129, 115103. https://doi.org/10.1016/j.bone.2019.115103
Vander Wyst, K. B., Hu, H. H., Peña, A., Olson, M. L., Bailey, S. S., & Shaibi, G. Q. (2021). Bone marrow adipose tissue content in Latino adolescents with prediabetes and obesity. Obesity (Silver Spring, Md.), 29(12), 2100–2107. https://doi.org/10.1002/oby.23279
da Silva, S. V., Renovato-Martins, M., Ribeiro-Pereira, C., Citelli, M., & Barja-Fidalgo, C. (2016). Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obesity (Silver Spring, Md.), 24(12), 2522–2532. https://doi.org/10.1002/oby.21660
Tencerova, M., Frost, M., Figeac, F., Nielsen, T. K., Ali, D., Lauterlein, J.-J.L., … & Kassem, M. (2019). Obesity-associated hypermetabolism and accelerated senescence of bone marrow stromal stem cells suggest a potential mechanism for bone fragility. Cell Reports, 27(7), 2050-2062.e6. https://doi.org/10.1016/j.celrep.2019.04.066
Dirat, B., Bochet, L., Dabek, M., Daviaud, D., Dauvillier, S., Majed, B., … & Muller, C. (2011). Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Research, 71(7), 2455–2465. https://doi.org/10.1158/0008-5472.CAN-10-3323
Wang, Y. Y., Attané, C., Milhas, D., Dirat, B., Dauvillier, S., Guerard, A., … Muller, C. (2017). Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight, 2(4). https://doi.org/10.1172/jci.insight.87489
Laurent, V., Toulet, A., Attané, C., Milhas, D., Dauvillier, S., Zaidi, F., … & Muller, C. (2019). Periprostatic adipose tissue favors prostate cancer cell invasion in an obesity-dependent manner: Role of oxidative stress. Molecular cancer research: MCR, 17(3), 821–835. https://doi.org/10.1158/1541-7786.MCR-18-0748
Zhang, M., Di Martino, J. S., Bowman, R. L., Campbell, N. R., Baksh, S. C., Simon-Vermot, T., … & White, R. M. (2018). Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discovery, 8(8), 1006–1025. https://doi.org/10.1158/2159-8290.CD-17-1371
Liu, H., He, J., Koh, S. P., Zhong, Y., Liu, Z., Wang, Z., … Yang, J. (2019). Reprogrammed marrow adipocytes contribute to myeloma-induced bone disease. Science Translational Medicine, 11(494), eaau9087. https://doi.org/10.1126/scitranslmed.aau9087
Trotter, T. N., Gibson, J. T., Sherpa, T. L., Gowda, P. S., Peker, D., & Yang, Y. (2016). Adipocyte-lineage cells support growth and dissemination of multiple myeloma in bone. The American Journal of Pathology, 186(11), 3054–3063. https://doi.org/10.1016/j.ajpath.2016.07.012
Fairfield, H., Dudakovic, A., Khatib, C. M., Farrell, M., Costa, S., Falank, C., … & Reagan, M. R. (2021). Myeloma-modified adipocytes exhibit metabolic dysfunction and a senescence-associated secretory phenotype. Cancer Research, 81(3), 634–647. https://doi.org/10.1158/0008-5472.CAN-20-1088
Hudak, C. S., Gulyaeva, O., Wang, Y., Park, S.-M., Lee, L., Kang, C., & Sul, H. S. (2014). Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Reports, 8(3), 678–687. https://doi.org/10.1016/j.celrep.2014.06.060
Fairfield, H., Costa, S., Falank, C., Farrell, M., Murphy, C. S., D’Amico, A., … & Reagan, M. R. (2020). Multiple myeloma cells alter adipogenesis, increase senescence-related and inflammatory gene transcript expression, and alter metabolism in preadipocytes. Frontiers in Oncology, 10, 584683. https://doi.org/10.3389/fonc.2020.584683
Boyd, A. L., Reid, J. C., Salci, K. R., Aslostovar, L., Benoit, Y. D., Shapovalova, Z., … & Bhatia, M. (2017). Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nature Cell Biology, 19(11), 1336–1347. https://doi.org/10.1038/ncb3625
Yang, S., Lu, W., Zhao, C., Zhai, Y., Wei, Y., Liu, J., … & Shi, J. (2020). Leukemia cells remodel marrow adipocytes via TRPV4-dependent lipolysis. Haematologica, 105(11), 2572–2583. https://doi.org/10.3324/haematol.2019.225763
Lu, W., Weng, W., Zhu, Q., Zhai, Y., Wan, Y., Liu, H., … & Shi, J. (2018). Small bone marrow adipocytes predict poor prognosis in acute myeloid leukemia. Haematologica, 103(1), e21–e24. https://doi.org/10.3324/haematol.2017.173492
Liu, H., Zhai, Y., Zhao, W., Wan, Y., Lu, W., Yang, S., … & Shi, J. (2018). Consolidation chemotherapy prevents relapse by indirectly regulating bone marrow adipogenesis in patients with acute myeloid leukemia. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 45(6), 2389–2400. https://doi.org/10.1159/000488225
Heydt, Q., Xintaropoulou, C., Clear, A., Austin, M., Pislariu, I., Miraki-Moud, F., … & Patel, B. (2021). Adipocytes disrupt the translational programme of acute lymphoblastic leukaemia to favour tumour survival and persistence. Nature Communications, 12(1), 5507. https://doi.org/10.1038/s41467-021-25540-4
Gazi, E., Gardner, P., Lockyer, N. P., Hart, C. A., Brown, M. D., & Clarke, N. W. (2007). Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. Journal of Lipid Research, 48(8), 1846–1856. https://doi.org/10.1194/jlr.M700131-JLR200
Shafat, M. S., Oellerich, T., Mohr, S., Robinson, S. D., Edwards, D. R., Marlein, C. R., … & Rushworth, S. A. (2017). Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood, 129(10), 1320–1332. https://doi.org/10.1182/blood-2016-08-734798
Panaroni, C., Fulzele, K., Mori, T., Siu, K. T., Onyewadume, C., Maebius, A., & Raje, N. (2022). Multiple myeloma cells induce lipolysis in adipocytes and uptake fatty acids through fatty acid transporter proteins. Blood, 139(6), 876–888. https://doi.org/10.1182/blood.2021013832
Lu, W., Wan, Y., Li, Z., Zhu, B., Yin, C., Liu, H., … & Shi, J. (2018). Growth differentiation factor 15 contributes to marrow adipocyte remodeling in response to the growth of leukemic cells. Journal of Experimental & Clinical Cancer Research, 37(1), 66. https://doi.org/10.1186/s13046-018-0738-y
Diedrich, J. D., Rajagurubandara, E., Herroon, M. K., Mahapatra, G., Hüttemann, M., & Podgorski, I. (2016). Bone marrow adipocytes promote the Warburg phenotype in metastatic prostate tumors via HIF-1α activation. Oncotarget, 7(40), 64854–64877. https://doi.org/10.18632/oncotarget.11712
Herroon, M. K., Diedrich, J. D., Rajagurubandara, E., Martin, C., Maddipati, K. R., Kim, S., … & Podgorski, I. (2019). Prostate tumor cell-derived il1β induces an inflammatory phenotype in bone marrow adipocytes and reduces sensitivity to docetaxel via lipolysis-dependent mechanisms. Molecular cancer research: MCR, 17(12), 2508–2521. https://doi.org/10.1158/1541-7786.MCR-19-0540
Herroon, M. K., Rajagurubandara, E., Hardaway, A. L., Powell, K., Turchick, A., Feldmann, D., & Podgorski, I. (2013). Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget, 4(11), 2108–2123. https://doi.org/10.18632/oncotarget.1482
Tabe, Y., Yamamoto, S., Saitoh, K., Sekihara, K., Monma, N., Ikeo, K., … & Andreeff, M. (2017). Bone marrow adipocytes facilitate fatty acid oxidation activating AMPK and a transcriptional network supporting survival of acute monocytic leukemia cells. Cancer Research, 77(6), 1453–1464. https://doi.org/10.1158/0008-5472.CAN-16-1645
Ehsanipour, E. A., Sheng, X., Behan, J. W., Wang, X., Butturini, A., Avramis, V. I., & Mittelman, S. D. (2013). Adipocytes cause leukemia cell resistance to L-asparaginase via release of glutamine. Cancer Research, 73(10), 2998–3006. https://doi.org/10.1158/0008-5472.CAN-12-4402
Morris, E. V., Suchacki, K. J., Hocking, J., Cartwright, R., Sowman, A., Gamez, B., … & Edwards, C. M. (2020). Myeloma cells down-regulate adiponectin in bone marrow adipocytes via TNF-alpha. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 35(5), 942–955. https://doi.org/10.1002/jbmr.3951
Caers, J., Deleu, S., Belaid, Z., De Raeve, H., Van Valckenborgh, E., De Bruyne, E., … & Vanderkerken, K. (2007). Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia, 21(7), 1580–1584. https://doi.org/10.1038/sj.leu.2404658
Liu, Z., Xu, J., He, J., Liu, H., Lin, P., Wan, X., … Yang, J. (2015). Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget, 6(33), 34329–34341. https://doi.org/10.18632/oncotarget.6020
Yu, W., Cao, D.-D., Li, Q.-B., Mei, H.-L., Hu, Y., & Guo, T. (2016). Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget, 7(52), 86075–86086. https://doi.org/10.18632/oncotarget.13342
Templeton, Z. S., Lie, W.-R., Wang, W., Rosenberg-Hasson, Y., Alluri, R. V., Tamaresis, J. S., … & King, B. L. (2015). Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia, 17(12), 849–861. https://doi.org/10.1016/j.neo.2015.11.005
Chen, G.-L., Luo, Y., Eriksson, D., Meng, X., Qian, C., Bäuerle, T., … Bozec, A. (2016). High fat diet increases melanoma cell growth in the bone marrow by inducing osteopontin and interleukin 6. Oncotarget, 7(18), 26653–26669. https://doi.org/10.18632/oncotarget.8474
Guérard, A., Laurent, V., Fromont, G., Estève, D., Gilhodes, J., Bonnelye, E., … & Muller, C. (2021). The chemokine receptor CCR3 is potentially involved in the homing of prostate cancer cells to bone: Implication of bone-marrow adipocytes. International Journal of Molecular Sciences, 22(4), 1994. https://doi.org/10.3390/ijms22041994
Hardaway, A. L., Herroon, M. K., Rajagurubandara, E., & Podgorski, I. (2015). Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clinical & Experimental Metastasis, 32(4), 353–368. https://doi.org/10.1007/s10585-015-9714-5
Larsson, S. C., & Wolk, A. (2007). Body mass index and risk of multiple myeloma: A meta-analysis. International Journal of Cancer, 121(11), 2512–2516. https://doi.org/10.1002/ijc.22968
Larsson, S. C., & Wolk, A. (2008). Overweight and obesity and incidence of leukemia: A meta-analysis of cohort studies. International Journal of Cancer, 122(6), 1418–1421. https://doi.org/10.1002/ijc.23176
Castillo, J. J., Reagan, J. L., Ingham, R. R., Furman, M., Dalia, S., Merhi, B., … & Mitri, J. (2012). Obesity but not overweight increases the incidence and mortality of leukemia in adults: A meta-analysis of prospective cohort studies. Leukemia Research, 36(7), 868–875. https://doi.org/10.1016/j.leukres.2011.12.020
Dhakal, P., Lyden, E., Lee, A., Michalski, J., Al-Kadhimi, Z. S., Maness, L. J., … & Bhatt, V. R. (2020). Effects of obesity on overall survival of adults with acute myeloid leukemia. Clinical Lymphoma, Myeloma & Leukemia, 20(3), e131–e136. https://doi.org/10.1016/j.clml.2019.11.001
Butturini, A. M., Dorey, F. J., Lange, B. J., Henry, D. W., Gaynon, P. S., Fu, C., … & Carroll, W. L. (2007). Obesity and outcome in pediatric acute lymphoblastic leukemia. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 25(15), 2063–2069. https://doi.org/10.1200/JCO.2006.07.7792
Gong, Z., Agalliu, I., Lin, D. W., Stanford, J. L., & Kristal, A. R. (2007). Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer, 109(6), 1192–1202. https://doi.org/10.1002/cncr.22534
von Drygalski, A., Tran, T. B., Messer, K., Pu, M., Corringham, S., Nelson, C., & Ball, E. D. (2011). Obesity is an independent predictor of poor survival in metastatic breast cancer: Retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. International Journal of Breast Cancer, 2011, 523276. https://doi.org/10.4061/2011/523276
Lazar, I., Clement, E., Dauvillier, S., Milhas, D., Ducoux-Petit, M., LeGonidec, S., … & Nieto, L. (2016). Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: A novel mechanism linking obesity and cancer. Cancer Research, 76(14), 4051–4057. https://doi.org/10.1158/0008-5472.CAN-16-0651
Clement, E., Lazar, I., Attané, C., Carrié, L., Dauvillier, S., Ducoux-Petit, M., … Nieto, L. (2020). Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. The EMBO journal, 39(3), e102525. https://doi.org/10.15252/embj.2019102525
Evangelista, G. C. M., Salvador, P. A., Soares, S. M. A., Barros, L. R. C., da Xavier, F. H., & C., Abdo, L. M., … Gameiro, J. (2019). 4T1 mammary carcinoma colonization of metastatic niches is accelerated by obesity. Frontiers in Oncology, 9, 685. https://doi.org/10.3389/fonc.2019.00685
Yun, J. P., Behan, J. W., Heisterkamp, N., Butturini, A., Klemm, L., Ji, L., … Mittelman, S. D. (2010). Diet-induced obesity accelerates acute lymphoblastic leukemia progression in two murine models. Cancer Prevention Research (Philadelphia, Pa.), 3(10), 1259–1264. https://doi.org/10.1158/1940-6207.CAPR-10-0087
Funding
Work in our team is supported by the “Ligue Nationale contre le Cancer” (Équipe labélisée) and the “Institut National du Cancer” (INCa PLBio 2020–28).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hernandez, M., Shin, S., Muller, C. et al. The role of bone marrow adipocytes in cancer progression: the impact of obesity. Cancer Metastasis Rev 41, 589–605 (2022). https://doi.org/10.1007/s10555-022-10042-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10042-6