Abstract
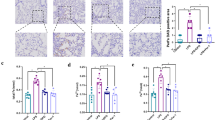
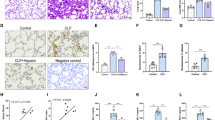
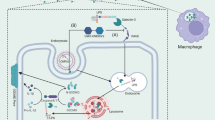
Vascular endothelial cell barrier disruption is a hallmark of sepsis-induced acute lung injury (ALI). Mesenchymal stem cells (MSCs)-based therapy has been regarded as a promising treatment for repairing injured lungs, and mitochondrial transfer was shown to be important for the therapeutic effects of MSCs. Here we investigated the ability of MSCs to modulate endothelial barrier integrity through mitochondrial transfer in sepsis-induced ALI. We found that mitochondrial transfer from MSCs to LPS-induced PMVECs through forming tunneling nanotubes (TNTs). Due to the inhibition of TNTs (using LAT-A), MSCs-mediated reparation on PMVECs functions, including cell apoptosis, MMP, ATP generation, TEER level and monolayer permeability of FITC-dextran were greatly inhibited. In addition, silencing of mitochondrial transcription factor A (TFAM) in MSCs could also partly inhibit the TNTs formation and aggravate the LPS-induced mitochondrial dysfunction and permeability barrier in PMVECs. Furthermore, the LPS-induced pulmonary edema and higher pulmonary vascular permeability were alleviated by MSCs while that of lung tissue bounced back after MSCs were pre-incubated by LAT-A and or down-regulation of TFAM. Therefore, we firstly revealed that regulation of TFAM expression in MSCs played a critical role to improve the permeability barrier of PMVECs by TNTs mediating mitochondrial transfer in sepsis-associated ALI. This study provided a new therapeutic strategy for the treatment of sepsis-induced ALI.








Similar content being viewed by others
Data availability
The data reported in this study are available from the corresponding author (yinhaiyan1867@126.com) upon reasonable request.
References
Ren Y et al (2095) [Research progress of extracellular vesicle microRNA in acute lung injury] –4352 (Print))
Zhang CN et al (2021) The role of extracellular vesicles in traumatic brain injury-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 321(5):L885–l891
Mowery NT, Terzian WTH, Nelson AC (2020) Acute lung injury. Curr Probl Surg 57(5):100777
Butt Y, Fau - Kurdowska A, Kurdowska TC, Fau A, Allen, Allen TCAcute Lung Injury: A Clinical and Molecular Review. (1543–2165(Electronic)).
Li N et al (2021) Major signaling pathways and key mediators of macrophages in acute kidney injury (review). Molecular Medicine Reports 23(6)
Jiang D et al (2016) Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis 7(11):e2467
Tammaro A et al (2019) TREM1/3 Deficiency Impairs Tissue Repair After Acute Kidney Injury and Mitochondrial Metabolic Flexibility in Tubular Epithelial Cells. Other 10
Gupta N et al (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179(3):1855–1863
Xu J et al (2007) Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice 293(1): p. L131–L141
Ahmad T et al Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy (1460–2075 (Electronic))
Dutra Silva J et al (2021) Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J 58(1)
Zhao M et al (2021) Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS Nano 15(1):1519–1538
Rustom A et al (2004) Nanotubular highways for intercellular organelle transport. Science 303(5660):1007–1010
Mohana Devi S et al (2021) Leber’s hereditary optic neuropathy: current approaches and future perspectives on mesenchymal stem cell-mediated rescue. Mitochondrion 60:201–218
Yao Y et al (2018) Connexin 43-Mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem Cell Reports 11(5):1120–1135
Sun W et al (2022) Highly pathogenic PRRSV-Infected alveolar Macrophages impair the function of Pulmonary Microvascular endothelial cells. Viruses 14(3)
Natarajan R, Northrop N, Yamamoto B (2017) Fluorescein Isothiocyanate (FITC)-Dextran Extravasation as a measure of blood-brain barrier permeability. Curr Protoc Neurosci 79:p. 9.58.1–9.58.15
Chen Y et al (2021) Moesin Is a Novel Biomarker of Endothelial Injury in Sepsis. J Immunol Res 2021:p. 6695679
He J et al (2021) MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-Akt signaling pathway. Biomaterials 265:120448
Fu X et al (2019)Mesenchymal Stem Cell Migration and Tissue Repair. Cells 8(8)
Fernández-Francos S et al (2021) Mesenchymal stem cell-based therapy as an alternative to the treatment of Acute Respiratory Distress Syndrome: current evidence and future perspectives. Int J Mol Sci 22(15)
Meng SS et al (2019) mTOR/STAT-3 pathway mediates mesenchymal stem cell-secreted hepatocyte growth factor protective effects against lipopolysaccharide-induced vascular endothelial barrier dysfunction and apoptosis. J Cell Biochem 120(3):3637–3650
Meng SS et al (2021) mTORC2 Activation Mediated by Mesenchymal Stem Cell-Secreted Hepatocyte Growth Factors for the Recovery of Lipopolysaccharide-Induced Vascular Endothelial Barrier. Stem Cells Int 2021:p. 9981589
Darley-Usmar V (2004) The powerhouse takes control of the cell; the role of mitochondria in signal transduction. Free Radic Biol Med 37(6):753–754
Bongard RD, Townsley MI, Merker MP (2015) The effects of mitochondrial complex I blockade on ATP and permeability in rat pulmonary microvascular endothelial cells in culture (PMVEC) are overcome by coenzyme Q1 (CoQ1). Free Radic Biol Med 79:69–77
Islam MN et al (2012) Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18(5):759–765
Michaeloudes C et al (2021) Study of mesenchymal stem cell-mediated mitochondrial transfer in in Vitro Models of oxidant-mediated airway epithelial and smooth muscle cell Injury. Methods Mol Biol 2269:93–105
Acknowledgements
None.
Funding
This study was supported by grants from the National Natural Science Foundation of China (NO. 82072232 and NO.81871585), the Natural Science Foundation of Guangdong Province (NO. 2018A030313058), the Medical Scientific Research Foundation of Guangdong Province, China (NO.A2021176) and the Planned Science and Technology project of Guangzhou, China (NO. 201804010308).
Author information
Authors and Affiliations
Contributions
Haiyan Yin participated in study design. Feng Zhang and Xinglong Zheng made the experiments. Fengzhi Zhao made the statistical analysis. Longzhu Li and Lijun Li assisted with data analysis. Feng Zhang prepared the manuscript, and Yinlong Ren and Haiyan Huang assisted with literature search. Haiyan Yin provided the financial support.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the animal experiments were undertaken in accordance with the National Institute of Health Guide for the Care and Use of laboratory Animals with approval from the Animal Ethics Committee of the First Affiliated Hospital of Jinan university.
Consent for publication
All the authors have read the manuscript and agreed to submit the paper to the journal.
Competing interests
All authors declare having no conflict of interest related to this research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Feng Zhang and Xinglong Zheng Contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, F., Zheng, X., Zhao, F. et al. TFAM-Mediated mitochondrial transfer of MSCs improved the permeability barrier in sepsis-associated acute lung injury. Apoptosis 28, 1048–1059 (2023). https://doi.org/10.1007/s10495-023-01847-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01847-z