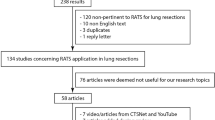
Summary
Indications for the use of RATS vary between the facilities but correspond as far as possible to other minimally invasive surgical findings. In general, RATS is currently a therapeutic option for the management of early-stage NSCLC without mediastinal lymph node involvement in oncological surgery, although depending on the planned intervention and the surgical facility, hilar lymph node involvement may be accepted
Similar content being viewed by others
Introduction
Since the introduction of minimally invasive procedures in thoracic surgery in the form of video-assisted thoracoscopy (VATS), it has now become widely established for the entire spectrum of thoracic interventions in addition to open thoracic surgery. Its advantages for the patient, such as reduced surgical trauma, shorter hospital stay, and less postoperative pain and complications, have been shown many times in the literature [1,2,3].
However, due to rigid instruments with altered maneuverability and orientation, limited haptics and visualization in the context of VATS, thoracotomy retains its status as the gold standard for thoracic surgery.
With the introduction of the first surgical robots in 2000, first and foremost the daVinci ® operating system from Intuitive Surgical (Sunnyvale, CA), they were used for thoracic surgery in the form of robot-assisted thoracoscopy (RATS) [4]. The safe use of RATS was demonstrated in the years that followed [5, 6]. At present, no significant differences between RATS and VATS with respect to clinical outcome have been shown [7, 8]. It is hoped that with the technical capabilities of the robot (3DHD optics, wrist-like range of motion of the EndoWrist® instruments, tremor reduction, intuitive translation of the movements of the console surgeon by the robot) and corresponding experience of the user, it will be possible to overcome the limitations of VATS. Due to the complete loss of haptics in the context of RATS, there are indications that this can be replaced by an evolving optical feedback in the sense of an optical haptic [9,10,11]. Due to the high costs associated with the acquisition of the daVinci® and the necessity of establishing a new surgical technique, its use has so far been reserved for specialized centers. It is reserved for use in thoracic surgery in Germany [11].
Materials and methods
The daVinci Si® surgical system
The daVinci Si® (Intuitive Surgical, Sunnyvale, CA, USA) operating system consists of the surgeon console and the patient cart. The surgeon console has a 3DHD visualization system and touch controls for video, audio, and system settings. From the console, the surgeon controls the robotic arms via fingertip master controls and foot pedals. The patient is positioned under the patient cart, which carries three robotic arms and one camera arm, and the movements of the surgeon at the console are transmitted to the wristed instruments on the robotic arms, which have a range of motion similar to that of the human hand. The camera system with 0/30-degree endoscope delivers highly magnified images (up to 10 × magnification) to the console surgeon and the rest of the surgical team.
Introduction to RATS
The introduction to the handling of the system, which is guided by Intuitive Surgical, initially consists of online modules with simulations and training in the technology and application of the robotic arms, and a final online examination. This is followed by advanced courses and exercises on cadavers. The training was completed by clinical observations and the accompaniment of the first five operations by a proctor and a staff member of Intuitive Surgical.
In our section, two thoracic surgery teams were trained, so that initially two console surgeons were qualified, who were able to combine their professional expertise on site. Two table assistants were available on an alternating basis. The first 10 procedures were performed by a largely consistent team of a console surgeon, assistant, anesthetist, and robotic operating room nurse, so that workflows were optimized more quickly and experience could be exchanged. In the initial start-up phase (n = 30), it was preferred, where possible, to combine the same console surgeons and assistants, in order to enable faster team adjustment to each other and harmonization of procedures, as well as better exchange of experience between two constant team setups, thus ensuring greater pre- and intraoperative safety. After the proctor had personally supervised the procedures in the first week, he was included by conference call for the subsequent operations. Sketches on the monitor allowed details to be discussed and graphically coordinated. By recording the operations and subsequent joint evaluation, this system of mutual professional development could be further supported.
Preoperative assessment and indication
The indication for a robot-assisted intervention was determined according to the criteria for conventional video-assisted thoracoscopy. Patients had to have sufficient cardiopulmonary reserve, whereby an absolute preoperative FEV1 (forced expiratory volume in 1 s) of >1.2 L was used as the limit value for the indication test for pulmonary resections. The indication assessment was performed by the console surgeon on the basis of a current chest computerized tomography (CT) scan. In case of proven or clinical suspicion of non-small-cell lung cancer (NSCLC) or carcinoid, a positron-emission tomography (PET) scan for N‑status assessment was initiated. Initially, contraindications were lesions with a diameter >3 cm, infiltration of the thoracic wall, or intrusion into the bronchial system, as well as centrally located tumors.
Patient positioning, anatomical sketch, and placement of the ports
The patient was positioned laterally with the arms extended. After checking the systems, a topographical clinical examination was carried out and a sketch was made of the layout of the four incisions on the thorax (Fig. 1). The daVinci Si® from Intuitive Surgical (Sunnyvale, CA) was used for all procedures. The operations were all performed using the three-arm technique, with two 8‑mm ports for robot arms 1 and 2 and one 12-mm port for the 10 mm 0‑degree optic. Via the assistant port the table assistant, in a single-port technique, performs certain tasks such as the insertion and removal of preparation swabs, compression of the lung parenchyma, suction of fluids, stapling of parenchyma, vessels, and bronchus, removal of preparations in the extraction bag as well as the intraoperative exchange of daVinci® instruments. The ports were placed under additional local anesthesia, starting with the assistant port at the height of the eighth intercostal space (ICS) anterolaterally (anterior axillary line) with a 2-cm incision. With this thoracocentesis, the incisions for the camera port (12 mm) in the posterior axillary line, the posterior daVinci® port (8 mm; second robotic arm) posterior to the inferior angle of the scapula, and the anterior daVinci® port (8 mm; first robotic arm) anterolateral were created with camera guidance (Fig. 1). The three ports for the robot arms were placed in the same ICS, on the left mostly in the sixth ICS and on the right in the fifth ICS, with a minimum distance of approximately 8 cm between the ports to avoid collisions of the robotic arms. The correct penetration depth of the trocars according to the markings placed on the trocars is important to avoid trauma to the thoracic wall and hematoma formation.
Intraoperative management
The specimens were removed by means of an extraction bag and under camera guidance a chest drain was placed via the assistant port. The instruments were removed under visual control and after a final assessment of intrathoracic conditions, the camera was removed. In the case of intraoperative complications which prevented continuation of the procedure by means of a robot, a thoracotomy was performed.
Special anesthesiologic features
All patients were ventilated with a double-lumen tube, whereby after incision a single-lung ventilation with partial CO2 insufflation via daVinci® port was performed (flow 5 L/min, 6–8 mm Hg) in order to create better visibility in the pleural cavity. Due to the CO2 insufflation, a close consultation of the team was necessary at the beginning of the procedure in order to detect possible mediastinal effects (e.g., hypotension, tachycardia), some of which occurred but did not pose any further problems for the surgical procedure. Due to the fact that the robotic patient module is connected above the patient’s head, an uncomfortable situation can arise for the anesthetist, as access to the tube is difficult. Access to the tube was secured via a tunnel, as far as possible, thus providing the possibility for manipulation and optimization during the operation. After positioning the patient for surgery, the tube position is checked again by means of bronchoscopy. Ventilation and the depth of anesthesia were ensured via extension tubes.
Postoperative management
The patients were, where possible, extubated in the operating room and then transferred to the normal ward. In the case of intraoperative complications, circulatory instability, or an age >80 years, transfer to the intensive care unit (ICU) was arranged for at least one day postoperatively. Early postoperative mobilization, short drainage times, adequate analgesia management, and prompt discharge with return to previous activity levels were the objectives. Regular chest x‑rays were performed. Thoracic drainage with a suction height of 12 cm H2O was removed from the first postoperative day in the absence of leakage and with a discharge rate of <50 ml. Patients were discharged, where possible, after removal of the chest drain and observation of good overall condition also from the first postoperative day.
Results
The patient group
Between 09/2015 and 02/2017, 58 RATS interventions were performed in 53 patients. 31 patients (58.9%) were male, mean age was 57 years (range: 18 to 85 years). The median age was 61 years for the male patients and 59 years for the female patients.
27 patients (51%) had a positive nicotine history at the time of surgery or in the past, of whom 8 patients had chronic obstructive pulmonary disease (COPD). 25 patients (47%) had some form of arterial hypertension and in 19 cases (36%) had other previous cardiovascular diseases. In 19 patients there was a current or past history of malignant tumors. In 13 cases (25%) other relevant comorbidities were present, including secondary pulmonary arterial hypertension, Child–Pugh B (ethyl toxicity) liver cirrhosis, hyperthyroidism, bronchial asthma, past successful liver transplantation, existing immunosuppression, and Churg–Strauss syndrome. 23 (43%) patients had an American Society of Anesthesiology (ASA) score of III. The FEV1 score was determined in 51 cases, with the two missing values accounted for by the respective indications, i.e., spontaneous pneumothorax in an 18-year-old and recurrent pleural effusions in a patient with ovarian cancer.
The surgical indications included clarification or resection of an unclear pulmonary mass in 30 cases (51.7%), histologically confirmed bronchial carcinoma (NSCLC) in 3 cases (5.1%), hyperhidrosis in 6 patients (19% in 11 interventions), mediastinal masses (tumor, abscess, cyst) and pleural effusions in 4 cases (6.9%), and pleural lesions in 2 patients (3.5%). Finally, the histological clarification of pulmonary fibrosis in one case (5.2%) and of bullous emphysema after spontaneous pneumothorax in another case, and pneumolysis plus decompression of a nerve after serial rib fracture are coded under rare indications in 3 cases (5.2%).
The procedures performed included 15 lobectomies, 5 segmental resections, 6 wedge resections, 4 excisions of mediastinal masses, 5 pleurectomies, 2 decortications, and 11 sympathectomies for hyperhidrosis—both sides in 5 patients in one surgical session. Each side was evaluated as a separate procedure, as each side had to be positioned, docked, and closed independently of the other side. Some pulmonary resections also underwent pleurectomy and/or decortication, but this was associated with the pulmonary resection and only evaluated as a separate intervention if performed as the sole surgical measure. In 15 lobectomies, 7 upper lobe resections, 7 lower lobe resections, and one middle lobe resection were performed. The excision of the mediastinal mass was performed exclusively from the right side. A total of 62.1% of the interventions were performed on the right side, whereby the selection of patients with regard to the side to be operated on was arbitrary. In 15 patients a radical lymphadenectomy was additionally performed and in 14 cases sampling of only one lymph node was performed. The exact number of lymph nodes sampled was not recorded.
Surgery and docking times
The average surgery times were broken down by procedure. For lobectomies the mean operating time was 157 min (range: 72–229 min), for segment resections 168 min (range: 125–202 min), for wedge resections 74 min (range: 40–104 min), and for the remaining procedures the mean operating time was in the range of 36 to 112 min.
The mean docking time for the first 10 procedures was 25.4 min, which decreased to 16.2 min (10th–20th procedure), 14.4 min (20th–30th procedure), and to 13.57 min for the last 28 procedures. In more than 58.62% of the operations (34 procedures) the docking time was less than 16 min.
Intraoperative complications and conversions
In the course of surgery, no intraoperative complications in the sense of large uncontrollable bleeds occurred, which would have made an emergency conversion necessary. The robot was deliberately undocked during two lobectomies because of intraoperative need, and the operations converted to an anterolateral thoracotomy (conversion rate 3.4%). In the first case, it proved impossible to adequately oxygenate the patient by single-lung ventilation and the decision was made to convert to open surgery to complete the upper lobe resection. In the second case, difficulties arose intraoperatively because the lymph nodes to be removed were tightly adhered to the left pulmonary artery and it became necessary to convert to open surgery in order to have better control in case of possible bleeding. Intraoperative mortality was 0%.
Postoperative management
Patients were transferred either directly to the normal ward (23, 43%) or, depending on individual risk and complexity of the operation, to the high dependency unit (HDU) or intensive care unit (ICU) for monitoring (30, 57%). Extubation mainly took place in the operating room, with a few exceptions occurring in the ICU on the first postoperative day. Two patients required catecholamine after surgery, but both were catecholamine-free during the first postoperative day. The mean length of stay for postoperative patients in the ICU was 1.37 days, with a maximum stay of 4 days. Patients were mobilized as early as possible and the drains were removed within a period of 2 ± 1 days. In 2 patients the drainage was left in place for more than 3 days due to prolonged pleural effusion. All patients underwent postoperative radiographic and other follow-up examinations.
Postoperative morbidity and mortality
In 53 patients the postoperative complication rate was 32.3%. When regarding just the operated side (n = 58), the postoperative complication rate was 29.3% (17/58), with postoperative chest x‑ray findings including mantle pneumothorax in 8 cases and soft tissue emphysema in 3 patients. However, these did not represent an indication for treatment and resolved over time. Four patients had postoperative pleural effusions, one of which was progressive and was revised in the context of an existing hypoalbuminemia. After excision of a mediastinal abscess, a pleural empyema developed on the access side, which was surgically repaired in stages via thoracotomy. In addition, one patient showed a persistent pneumothorax following reoperation with RATS (after successful liver transplantation), which was also treated surgically. The revision rate was 5.2% (3/58) and mortality during the postoperative stay as well as the 30-day mortality was 0%.
Postoperative stay
For the 53 patients the mean postoperative stay was 5.04 days (range: 1–27 days). 43 (81%) patients were discharged within the first week after surgery and 10 (19%) patients had a postoperative stay of more than 6 days. The discharge of patients on the first postoperative day, with early removal of the chest tube, was done according to the principle of fast-track management.
The stay of 27 days was attributed to the aforementioned case of hypoalbuminemia with development of a progressive pleural effusion and revision of the operated side by single-port VATS on the left. In addition, during the postoperative stay of this patient, the rare diagnosis of pulmonary histoplasmosis was made while investigating a suspected tuberculosis.
Discussion
The advantages for the patient of minimally invasive thoracic surgery in the form of VATS over thoracotomy have been described many times in recent years. They include reduced surgical trauma, shorter hospital stays, and fewer postoperative complications. Nevertheless, thoracotomy remains the gold standard for thoracic surgery. The reasons for this are numerous: limited haptic feedback, rigid instruments with contra intuitive eye and hand coordination in combination with limited visual and spatial accessibility, as well as the uncertainty, in the case of major bleeds, of being able to react quickly and adequately.
In our clinic, the different forms of VATS (single- and multiport) are regularly applied. In an effort to further minimize intraoperative trauma, robot-assisted surgery brings new possibilities. Although the haptic or tactile feedback is further limited, the position of the surgeon at the console in conjunction with the three-dimensional representation of the intracavitary environment, the 10 × magnification, and the extended range of motion of the EndoWrist® instruments with tremor reduction provides a more natural orientation and maneuverability. A transformation of the tactile haptics into optical feedback is occasionally shown [9,10,11]. There is no international consensus on the number of robot arms used, the placement of the ports, or the creation of an assistant port (access/utility incision). The variation in application of the system is due to differences in the respective proctor, the degree of experience with minimally invasive techniques, and the individual spatial awareness and preferences of the surgeon. Our own technique was modified according to the ideas of the console surgeons in the sense of a slightly modified port placement. A fourth robotic arm is dispensed with and an assistant port is attached, whereby the assistant at the table can provide additional safety in case of complications.
Single-port expertise require a hybrid technique to support the console surgeon. Indications for the use of RATS also vary between the facilities but correspond as far as possible to other minimally invasive surgical findings. In general, RATS is currently a therapeutic option for the management of early-stage NSCLC without mediastinal lymph node involvement in oncological surgery, although depending on the planned intervention and the surgical facility, hilar lymph node involvement may be accepted. The clinical introduction of RATS has generated a range of lighter, less complex procedures. This has increased the safety as well as the surgical success in using the system. In the evaluation of RATS for everyday clinical use, the associated operating times, conversion rates, postoperative complication rates, and the learning curve derived from these are widely discussed. Authors have already pointed out that experienced VATS surgeons need about six RATS procedures in order to have surgery times and length of stay similar to their VATS procedures. However, this does not apply to surgeons inexperienced with VATS [12]. The mean operating time (cut-suture time) for the 15 lobectomies performed was 157 min (range: 72–229 min). Excluding the two converted lobectomies, the mean operating time was 159 min with a corresponding conversion rate of 13.3% for the lobectomies (2/15; 13.3%). In the literature for VATS lobectomy, the median procedure length is described with 130 min when performed by an experienced surgical team [13]. As one of the reasons for this, the not yet completed learning curve must be assumed.
In the converted procedures, an anterolateral thoracotomy was performed due to tightly adhered lymph nodes in one case and difficult lung ventilation in the other. Postoperative complications included frequently observed findings.
One of the most important questions regarding robotic-assisted surgery is without doubt how it performs in clinical practice alongside the established VATS. This is important primarily due to the significantly increased cost associated with RATS. Kneuertz et al. analyzed a cohort of 697 patients (269 robotic, 161 VATS, and 240 open) with exactly this focus. The pure surgery costs differed significantly (9912 $ robotic, 9491 $ VATS, and 8689 $ conventional). In contrast, the postoperative times were significantly shorter for the minimally invasive procedures (3.8 days robotic, 3.8 days VATS, and 5.4 days conventional). Interestingly, in the propensity score-adjusted analysis there were no significant differences in direct hospital costs (17,223 $ robotic, 17,260 $ VATS, and 18,075 $ conventional). The authors conclude that the increased surgical costs can be almost completely compensated by a reduction of the postoperative costs. It must be noted that the available data cannot be transferred directly to the German health system. However, the underlying mechanism of reducing postoperative costs, due to fewer complications and shorter hospital stay, is an additional aspect in this partly emotional discussion [14].
In this study the mean postoperative stay was 5.4 days. This is congruent with the postoperative stays described in the literature, which were significantly shorter for RATS in comparative studies between VATS and RATS lobectomy [15].
Several multi-institutional analyses as well as more recent meta-analyses over the past few years with a correspondingly large patient population allow for a better comparability of the two minimally invasive methods.
With the exception of the longer operation times with RATS, no significant differences between the two techniques were found [7, 11].
The information in the literature on the exact reasons for the longer operation times with the use of RATS is minimal; there are no data on docking times and longer operation times are mostly justified by lack of experience with the system. In our opinion, all in all, one should expect an additional 20–30 min of technical management.
With regard to the learning curve, there are indications that the application of RATS can be learned faster than VATS, which has been documented to have a very steep learning curve within 50–200 cases [16,17,18].
From our point of view, all in all, RATS offers a new opportunity for patients, a safe, comfortable, and less painful procedure that is recommended in the field of thoracic surgery. For surgeons with existing expertise in minimally invasive thoracic surgery, RATS is relatively easy to learn and apply to appropriate indications, with a flatter learning curve than for VATS. The corresponding differences and anatomical peculiarities can be learnt through minor operations up to oncological resection of small malignant flap-limited tumors. A systematic introduction of the system into the surgical routine is essential to ensure intraoperative safety. Surgeons should go through a program of systematic learning and preparation, which should include theoretical and technical instructions, exercises in the laboratory, observations, and cooperation with experienced RATS colleagues. The use of the new system should always be based on the demand for minimally invasive surgery. The purchase of a robot by a large center is more favorable, considering the potential use by more than one specialty.
It can be expected that new results will emerge in the next few years, especially with regard to the treatment of other tumor stages using RATS.
According to Steinert and Egberts (personal communication), the assessment of how many robotic arms the thoracic surgeon needs in combination with the usability and optical haptics is in line with the continuous development of RATS. In 2019, Durand et al. [19] showed another option for the three-arm technique with integration of the camera on the daVinci Xi® system, which offers a dramatic advantage for more complex procedures.
Vision in Europa
The first robotic program in Europe was documented in Italy by Professor F Melfi [4]. This initial experience was conducted with the first-generation system, the daVinci Standard®. This system was not as capable as future generations and had only three arms. The arrival of the third and fourth generations of the system (Da Vinci Si® and Xi®) combined with instruments designed for thoracic surgery has triggered a boom in robotic thoracic programs, as the versatility of four arms was more compliant with thoracic surgery specificities (multisite surgery, sharp vessel dissection, N1 and N2 node harvesting, moving target, etc.).
Currently, there are three centers for thoracic surgery in Europe, one in Italy (Pr. F Melfi, Pisa), one in France (Dr. M Durand, Paris), and one in Germany dedicated to mediastinal surgery (Dr. J Rueckert, Berlin).
Europe has a strong surgical culture when it comes to the treatment of cancer. Thus, we perform surgery from stage I (lobar and sublobar resections) to stage IV lung cancer (lobectomy, bilobectomy, pneumonectomy, sleeve resections) through multimodal treatment protocols for advanced cancer. Therefore, surgical skills are key.
So-called robotic surgery is about telemanipulation, immersive enhanced vision, and wristed surgery. The technical revolution in this area concerns both vision and control. Robotic techniques vary from three arms with assistant utility incision without capnothorax to fully endoscopic four-arm with capnothorax approaches. Thus, it should be a requirement to specify the technique used in order not to skew the results and to be able to apply relevant comparisons [19].
The most advanced robotic approach, i.e., four-arm totally endoscopic, compares favorably to open surgery steps and strategy and is currently the most taught and widely used in Europe. The arrival of the Xi® system in 2014 corresponded to the growth of this technique. This approach is of great interest especially for advanced procedures such as bronchial sleeve resections [20, 21] or sublobar resections. The three wristed instruments are particularly effective for sublobar dissection, target stabilization, exposure, and node harvesting. Resecting lobar lymph nodes is a tricky, more delicate procedure compared to the resection of mediastinal nodes, as the closely associated vascular structures must remain intact.
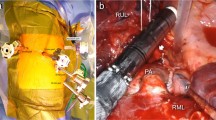
At present, the training process is mainly via peer-to-peer senior clinician skill advancement with support from experienced proctors and case observation in European referent centers. Thus, choice of technique must be made before starting (three-arm, four-arm, 0° vision, 30° vision, etc.), and the four-arm technique should be prioritized. The French four-arm technique [19] setup is shown in Fig. 2. The partial W shape offers non-conflict setting of the arms while allowing an extended control of the full chest cavity. It is clear that the development of robotic thoracic surgery has had an unprecedented effect by opening the doors of operating rooms across Europe, as surgeons share experiences and visit different centers, thus enhancing their vision and expertise.
Right-side port placement, camera port is on the scapula line eighth or seventh intercostal space (ICS), right-hand port seventh ICS, left-hand port tenth ICS, third hand 3 cm closer to the spine in the seventh ICS, and a 15-mm port access in the ninth ICS. The window shows the axis of the arms (arrows) and the triangle of working zone for the bedside assistant
As European thoracic surgeons, we still have questions to debate: How many cases per year could justify a safe robotic program? Should we standardize our techniques or approach? How can we integrate a robotic training program into university education? How can we manage and fund the cost of these expensive advanced clinical tools? And as fast as one high-tech solution appears, another is hard on its heels, so how can we keep ahead of the curve, strengthen our skills individually or as a community, including telementoring, robotic 8‑mm stapling, or robotic adapted vascular clamping?
Change history
28 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10353-021-00743-7
References
Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–78. https://doi.org/10.1016/j.jtcvs.2009.08.026.
Whitson BA, Andrade RSM, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83:1965–70. https://doi.org/10.1016/j.athoracsur.2007.01.049.
Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg. 2009;138:419–25. https://doi.org/10.1016/j.jtcvs.2009.04.026.
Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg. 2002;21:864–7. https://doi.org/10.1016/s1010-7940(02)00102-1.
Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg. 2012;143:383–9. https://doi.org/10.1016/j.jtcvs.2011.10.055.
Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014;97:236–42. https://doi.org/10.1016/j.athoracsur.2013.07.117.
Louie BE, Wilson JL, Kim S, et al. Comparison of video-assisted thoracoscopic surgery and robotic approaches for clinical stage I and stage II non-small cell lung cancer using the society of thoracic surgeons database. Ann Thorac Surg. 2016;102:917–24. https://doi.org/10.1016/j.athoracsur.2016.03.032.
Yang HX, Woo M, Sima CS, et al. Long-term survival based on the surgical approach to lobectomy for clinical stage I non-small-cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg. 2017;265:431–7. https://doi.org/10.1097/SLA.0000000000001708.
Sandhaus T, Lübke A, Möller T, et al. Roboter-assistierte Thoraxchirurgie. Eine weitere endoskopische Operationsmöglichkeit. CHAZ. 2019;20:279–85.
Meccariello G, Faedi F, AlGhamdi S, et al. An experimental study about haptic feedback in robotic surgery: may visual feedback substitute tactile feedback? J Robot Surg. 2016;10:57–61. https://doi.org/10.1007/s11701-015-0541-0.
Möller T, Egberts J‑H, et al. Current status and evolution of robotic-associated thoracic surgery in Germany—results from a nationwide survey. J Thorac Dis. 2019;11(11):4807–15.
Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg. 2012;93:1598–604. https://doi.org/10.1016/j.athoracsur.2012.01.067.
Swanson SJ, Herndon JE, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802—a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993–7.
Kneuertz PJ, Singer E, D’Souza DM, et al. Hospital cost and clinical effectiveness of robotic-assisted versus video-assisted thoracoscopic and open lobectomy: a propensity score–weighted comparison. J Thorac Cardiovasc Surg. 2019;157:2018–2026.e2.
Li J‑T, Liu P‑Y, Huang J, et al. Perioperative outcomes of radical lobectomies using robotic-assisted thoracoscopic technique vs. video-assisted thoracoscopic technique: retrospective study of 1,075 consecutive p‑stage I non-small cell lung cancer cases. J Thorac Dis. 2019;11:882–91.
Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg. 2012;1:47–50. https://doi.org/10.3978/j.issn.2225-319X.2012.04.05.
Cheufou DH, Mardanzai K, Ploenes T, et al. Effectiveness of robotic lobectomy-outcome and learning curve in a high volume center. Thorac Cardiovasc Surg. 2019;67:573–7. https://doi.org/10.1055/s-0038-1639477.
Power AD, D’Souza DM, Moffatt-Bruce SD, et al. Defining the learning curve of robotic surgery: what does it take? Surg Endosc. 2019;33:3880–8. https://doi.org/10.1007/s00464-019-07035-y.
Durand M, Dabboura E, et al. Four-arm robotic lung resection versus muscle-sparing mini-thoracotomy; retrospective experience. J Thorac Dis. 2019;11(4):1433–42.
Cerfolio R, Louie BE, Farivar AS, et al. Consensus statement on definitions and nomenclature for robotic thoracic surgery. J Thorac Cardiovasc Surg. 2017;154:1065–9.
Durand M. Four-arm robotic sleeve right upper lobectomy. Ann Cardiothorac Surg. 2019;8(2):286–7.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Sandhaus, M. Durand, T. Möller, J.H. Egberts, and M. Steinert declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sandhaus, T., Durand, M., Möller, T. et al. Robotic surgery for thoracic surgery. Eur Surg 53, 142–148 (2021). https://doi.org/10.1007/s10353-020-00674-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-020-00674-9